This is a preprint.
Short-chain fatty acids are a key mediator of gut microbial regulation of T cell trafficking and differentiation after traumatic brain injury
- PMID: 39606443
- PMCID: PMC11601855
- DOI: 10.21203/rs.3.rs-5397327/v1
Short-chain fatty acids are a key mediator of gut microbial regulation of T cell trafficking and differentiation after traumatic brain injury
Update in
-
Short-chain fatty acids are a key mediator of gut microbial regulation of T cell trafficking and differentiation after traumatic brain injury.Exp Neurol. 2025 Oct;392:115349. doi: 10.1016/j.expneurol.2025.115349. Epub 2025 Jun 15. Exp Neurol. 2025. PMID: 40527418
Abstract
The gut microbiota has emerged as a pivotal regulator of host inflammatory processes after traumatic brain injury (TBI). However, the mechanisms by which the gut microbiota communicates to the brain in TBI are still under investigation. We previously reported that gut microbiota depletion (GMD) using antibiotics after TBI resulted in increased microglial activation, reduced neurogenesis, and reduced T cell infiltration. In the present study, we have demonstrated that intestinal T cells contribute to the pool of cells infiltrating the brain after TBI. Depletion or genetic deletion of T cells before injury reversed GMD induced reductions in post-TBI neurogenesis. Short-chain fatty acid supplementation increased T regulatory and T helper1 cell infiltration to the brain along with restoring neurogenesis and microglia activation after TBI with GMD. These data suggest that T cell subsets are essential cellular mediators by which the gut microbiota modulates TBI pathogenesis, a finding with important therapeutic implications.
Keywords: T cell-trafficking; T cells; Traumatic brain injury; gut microbiome; gut microbiota depletion; gut-brain axis; microglia; neurogenesis; neuroinflammation; short-chain fatty acids.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures

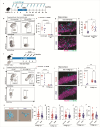


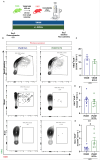

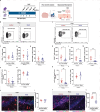
References
-
- Masel BE, DeWitt DS. Traumatic brain injury: a disease process, not an event. J Neurotrauma. 2010;27(8):1529–40. - PubMed
-
- Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nature reviews Neurology. 2013;9(4):231–6. - PubMed
-
- Treble-Barna A, Pilipenko V, Wade SL, Jegga AG, Yeates KO, Taylor HG, et al. Cumulative Influence of Inflammatory Response Genetic Variation on Long-Term Neurobehavioral Outcomes after Pediatric Traumatic Brain Injury Relative to Orthopedic Injury: An Exploratory Polygenic Risk Score. J Neurotrauma. 2020;37(13):1491–503. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

