New Plant Extracts Exert Complementary Anti-Hair Loss Properties in Human In Vitro and Ex Vivo Models
- PMID: 39606918
- PMCID: PMC11603400
- DOI: 10.1111/jocd.16616
New Plant Extracts Exert Complementary Anti-Hair Loss Properties in Human In Vitro and Ex Vivo Models
Abstract
Background: Hair loss is linked to dysfunction of the growth (anagen), regression (catagen) and rest (telogen) phases of the hair follicle (HF) cycle.
Aims: To evaluate the effects of a Silybum marianum extract (SME), manganese PCA (MnPCA), and a Lespedeza capitata extract (LCE) on markers of hair growth and anchorage in human follicle dermal papilla cells (HFDPCs), and to investigate the ability of a topical serum containing these active ingredients to improve HF growth in an ex vivo human scalp skin model.
Methods: In HFDPCs, we assessed receptor tyrosine kinase phosphorylation and Wnt/β-catenin pathway activation; quantified versican, vascular endothelial growth factor (VEGF) and Dickkopf-1 (DDK1) secretion; and evaluated 5α-reductase (5αR) activity. Using scalp skin biopsies from two female donors, we measured hair shaft elongation, analyzed hair matrix keratinocyte proliferation and apoptosis, and determined HF cycle stage and score.
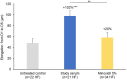
Results: Compared to untreated HFDPCs, SME upregulated phosphorylation of growth factor receptors (EGFR:1.9 × and PDGFR: 2.8 ×) and their downstream effectors (ERK, GSK3, Akt, and STAT: 1.2-2.0 ×); MnPCA enhanced versican (33.0 ×) and VEGF (3.3 ×) secretion, and stimulated the Wnt/β-catenin pathway (+80%); and LCE reduced DKK1 secretion (-72%) and 5αR activity (dihydrotestosterone/testosterone ratio: -60%). Compared to untreated scalp skin biopsies, the serum enhanced hair shaft elongation (+102%), and significantly prolonged the anagen phase by improving hair cycle scores and stimulating hair matrix keratinocyte proliferation (+58%).
Conclusions: SME, MnPCA, and LCE displayed complementary anti-hair loss properties. The serum combining these active ingredients may be useful in hair loss treatment.
Keywords: active ingredients; anchorage; dermal papilla; hair growth; hair loss; human hair follicle.
© 2024 LABORATOIRES PIERRE FABRE. Journal of Cosmetic Dermatology published by Wiley Periodicals LLC.
Conflict of interest statement
D.B., M.L., C.M., M.‐J.H., A.N., V.M., S.C., S.B.‐T., H.D., and N.C.R. are employees of Pierre Fabre Dermo‐Cosmétique & Personal Care, France, and as such received salaries during the period when the research described in this manuscript was conducted. D.B., H.D., and J.‐H.S. are co‐authors of patent WO/2021/023820, and M.L. is a co‐author of patent WO/2020/020791, both filed by Pierre Fabre Dermo‐Cosmétique, France. J.‐H.S. has a translational research contract with and has received personal and Institutional Research Fund from, Pierre Fabre Dermo‐Cosmétique & Personal Care.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

