Downregulation of Notch Signaling-Stimulated Genes in Neurovascular Unit Alterations Induced by Chronic Cerebral Hypoperfusion
- PMID: 39607309
- PMCID: PMC11603426
- DOI: 10.1002/iid3.70082
Downregulation of Notch Signaling-Stimulated Genes in Neurovascular Unit Alterations Induced by Chronic Cerebral Hypoperfusion
Abstract
Background: Chronic cerebral hypoperfusion (CCH) is a key contributor to vascular cognitive impairment (VCI) and is typically associated with blood-brain barrier (BBB) damage. This study investigates the pathological mechanisms underlying CCH-induced neurovascular unit (NVU) alterations.
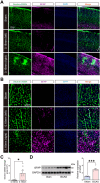
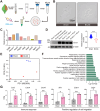
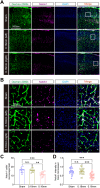
Methods: A mouse model of CCH was established using the bilateral common carotid artery stenosis (BCAS) procedure. Decreased cerebral blood flow (CBF) and impaired BBB integrity were assessed. Brain microvessel (BMV)-specific transcriptome profiles were analyzed using RNA-seq, supplemented with published single-cell RNA-seq data.
Results: RNA-seq revealed neuroinflammation-related gene activation and significant downregulation of Notch signaling pathway genes in BMVs post-BCAS. Upregulated differentially expressed genes (DEGs) were enriched in microglia/macrophages, while downregulated DEGs were prominent in endothelial cells and pericytes. Enhanced activation of vascular-associated microglia (VAM) was linked to neurovascular alterations.
Conclusion: CCH induces significant NVU changes, marked by microglia-associated neuroinflammation and Notch signaling downregulation. These insights highlight potential therapeutic targets for treating neuroinflammatory and vascular-related neurodegenerative diseases.
Keywords: Notch signaling pathway; brain microvessel; chronic cerebral hypoperfusion; microglial; neuroinflammation.
© 2024 The Author(s). Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

