Stereoselective block of the hERG potassium channel by the Class Ia antiarrhythmic drug disopyramide
- PMID: 39607488
- PMCID: PMC11604869
- DOI: 10.1007/s00018-024-05498-4
Stereoselective block of the hERG potassium channel by the Class Ia antiarrhythmic drug disopyramide
Abstract
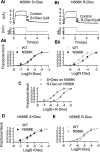
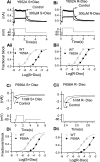
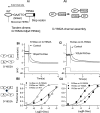
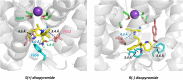
Potassium channels encoded by human Ether-à-go-go-Related Gene (hERG) are inhibited by diverse cardiac and non-cardiac drugs. Disopyramide is a chiral Class Ia antiarrhythmic that inhibits hERG at clinical concentrations. This study evaluated effects of disopyramide enantiomers on hERG current (IhERG) from hERG expressing HEK 293 cells at 37 °C. S(+) and R(-) disopyramide inhibited wild-type (WT) IhERG with IC50 values of 3.9 µM and 12.9 µM respectively. The attenuated-inactivation mutant N588K had little effect on the action of S(+) disopyramide but the IC50 for the R(-) enantiomer was ~ 15-fold that for S(+) disopyramide. The enhanced inactivation mutant N588E only slightly increased the potency of R(-) disopyramide. S6 mutation Y652A reduced S(+) disopyramide potency more than that of R(-) disopyramide (respective IC50 values ~ 49-fold and 11-fold their WT controls). The F656A mutation also exerted a stronger effect on S(+) than R(-) disopyramide, albeit with less IC50 elevation. A WT-Y652A tandem dimer exhibited a sensitivity to the enantiomers that was intermediate between that of WT and Y652A, suggesting Y652 groups on adjacent subunits contribute to the binding. Moving the Y (normally at site 652) one residue in the N- terminal (up) direction in N588K hERG markedly increased the blocking potency of R(-) disopyramide. Molecular dynamics simulations using a hERG pore model produced different binding modes for S(+) and R(-) disopyramide consistent with the experimental observations. In conclusion, S(+) disopyramide interacts more strongly with S6 aromatic binding residues on hERG than does R(-) disopyramide, whilst optimal binding of the latter is more reliant on intact inactivation.
Keywords: Disopyramide; Enantiomer; HERG; Long QT; QT interval; Stereoselectivity.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare that they have no actual or potential competing interests. Ethics approval and consent to participate: N/A. Consent for publication: N/A.
Figures


References
-
- Vandenberg JI, Walker BD, Campbell TJ (2001) HERG K+ channels: friend and foe. TIPS 22:240–246 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

