Radiation exposure and protection advice after [177Lu]Lu-DOTA-TATE therapy in China
- PMID: 39607652
- PMCID: PMC11604894
- DOI: 10.1186/s13550-024-01185-4
Radiation exposure and protection advice after [177Lu]Lu-DOTA-TATE therapy in China
Abstract
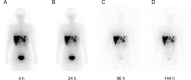
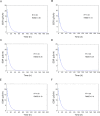
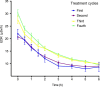
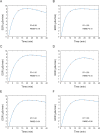
Background: We conducted a study on radiation exposure in patients with gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) treated with [177Lu]Lu-DOTA-TATE in China for the first time, aiming to provide guidance and reference for radiation protection in this regard. A total of 30 GEP-NENs patients who received [177Lu]Lu-DOTA-TATE therapy were recruited in the study. We measured the external dose rate (EDR) values of each patient during the injection and 0-6 h post-administration period, as well as the radiation dose (RD) values to healthcare nurses and the surrounding environment. We performed a double exponential curve fitting and estimated the RD to the public from patients discharged at different times after [177Lu]Lu-DOTA-TATE therapy.
Results: Among the 30 patients, 27 patients completed 4 cycles of [177Lu]Lu-DOTA-TATE treatments, the estimated RD to the public indicated that for adult family members, children above 10 years old, children aged 3-10 and coworkers of the patients, patients could begin daily contact at least 24 h, 48 h, 144 h and 192 h after injection to ensure that the total RD values after four treatments not exceed the limit. During the hospitalization of patients receiving [177Lu]Lu-DOTA-TATE, the cumulative dose received by the administering nurses and to the ward environment were both well below the national RD limits.
Conclusions: This study conducted a fitting analysis of the decay pattern of EDR values in GEP-NENs patients undergoing [177Lu]Lu-DOTA-TATE therapy, in order to establish guidelines for patient discharge timing and provide recommendations for radiation protection for the general public after patient discharge. Trial registration A Study Comparing Treatment With Lutetium[177Lu] Oxodotreotide Injection to Octreotide LAR in Patients With GEP-NETs, NCT05459844. https://clinicaltrials.gov/study/NCT05459844?cond=NCT05459844&rank=1 . Registered 5 July 2022.
Keywords: Dosimetry; GEP-NENs; Patient discharge; Radiation exposure; [177Lu]Lu-DOTA-TATE.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Ethics Committee of FUSCC (Approval No. 2210262-2). All patients provided written informed consent to participate in the study. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Consent for publication: Written informed consent for publication was obtained from all study participant. Competing interests: The authors declare that they have no competing interests.
Figures

References
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

