A Novel Y-Shaped Pegylated Recombinant Human Growth Hormone for Children With Growth Hormone Deficiency
- PMID: 39607675
- PMCID: PMC12261095
- DOI: 10.1210/clinem/dgae816
A Novel Y-Shaped Pegylated Recombinant Human Growth Hormone for Children With Growth Hormone Deficiency
Abstract
Context: Pegpesen is a novel Y-shape pegylated recombinant human growth hormone (rhGH) for once-weekly treatment of children with growth hormone deficiency (GHD).
Objective: This work aimed to evaluate the efficacy and safety of Pegpesen in children with GHD vs daily rhGH.
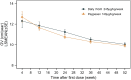
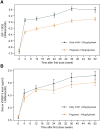
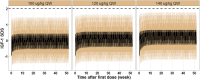
Methods: A multicenter, randomized, controlled phase 3 clinical trial was conducted at 23 centers in China with a duration of 52 weeks' treatment. There were 391 pediatric participants diagnosed with GHD. Participants were randomly assigned 2:1 to a weekly Pegpesen group (140 μg/kg/week) or a daily rhGH group (245 μg/kg/week) for 52 weeks. The primary end point was the growth velocity (GV) at 52 weeks, and the secondary end points mainly involved changes from baseline in height SD scores for chronological age and bone age (ΔHt SDS CA and ΔHt SDS BA).
Results: At 52 weeks, the least squares mean (LS means) of GV was 9.910 cm/year in the Pegpesen group and 10.037 cm/year in the daily rhGH group. The LS means difference between groups was -0.127 (95% CI, -0.4868 to 0.2332), confirming that weekly Pegpesen is noninferior to daily rhGH. The LS means of ΔHt SDS CA, ΔHt SDS BA, were similar across both groups (all P > .05). Safety profiles and adherence were comparable.
Conclusion: Pegpesen was noninferior to daily rhGH, with similar safety, lower dosage requirements, thus presenting a new therapeutic option for children with GHD.
Keywords: Y-shape pegylated rhGH; growth hormone; growth hormone deficiency; long-acting growth hormone; pediatric.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society.
Figures
References
-
- Ranke MB, Wit JM. Growth hormone — past, present and future. Nat Rev Endocrinol. 2018;14(5):285‐300. - PubMed
-
- Pampanini V, Deodati A, Inzaghi E, Cianfarani S. Long-acting growth hormone preparations and their use in children with growth hormone deficiency. Horm Res Paediatr. 2022;96(6):553‐559. - PubMed
-
- Geffner ME, Ranke MB, Wajnrajch MP; for the Pfizer International Growth Database Strategic Executive Committee, Strategic Advisory Board, and International Board . An overview of growth hormone therapy in pediatric cases documented in the Kabi international growth study (pfizer international growth database). Ann Pediatr Endocrinol Metab. 2024;29(1):3‐11. - PMC - PubMed
-
- Graham S, Weinman J, Auyeung V. Identifying potentially modifiable factors associated with treatment non-adherence in paediatric growth hormone deficiency: a systematic review. Horm Res Paediatr. 2018;90(4):221‐227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

