Palmitic acid and eicosapentaenoic acid supplementation in 3T3 adipocytes: impact on lipid storage and oxidative stress
- PMID: 39607809
- PMCID: PMC11610268
- DOI: 10.1080/13510002.2024.2430882
Palmitic acid and eicosapentaenoic acid supplementation in 3T3 adipocytes: impact on lipid storage and oxidative stress
Abstract
Objectives: Obesity is a worldwide public health problem, predisposing individuals to serious cardiovascular and metabolic complications such as type 2 diabetes mellitus. White adipose tissue serves as an important regulator of energy balance, and its expansion in obesity can trigger inflammatory reactions and oxidative stress, which can also lead to insulin resistance. Adipocytes, with a key role in regulating metabolic homeostasis, respond to increased calorie intake and altered fatty acid composition with hypertrophy or hyperplasia. Of particular interest are saturated fatty acids such as palmitic acid and omega-3 polyunsaturated fatty acids such as eicosapentaenoic acid (EPA), which have differential effects on adipocyte function and inflammation.
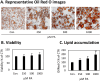
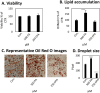
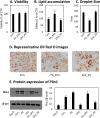
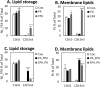
Methods: Using 3T3-L1 cells as a model for adipocytes, we evaluated the effects of PA and EPA on lipid accumulation, droplet size, and oxidative stress markers.
Results: We were able to show that EPA supplementation in 3T3 adipocytes does not lead to excessive lipid accumulation, but rather reduces the size of lipid droplets and also induces redox changes due to the unsaturated nature of EPA.
Discussion: These results emphasize the contrasting roles of PA and EPA and the importance of fatty acid composition in the regulation of adipocyte function.
Keywords: Obesity; adipocytes; eicosapentaenoic acid; fatty acids; lipid droplets; lipid storage; oxidative stress; palmitic acid.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
