Tlr7 drives sex differences in age- and Alzheimer's disease-related demyelination
- PMID: 39607927
- PMCID: PMC12396121
- DOI: 10.1126/science.adk7844
Tlr7 drives sex differences in age- and Alzheimer's disease-related demyelination
Abstract
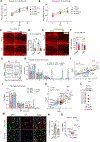
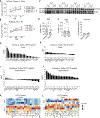
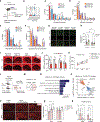
Alzheimer's disease (AD) and other age-related disorders associated with demyelination exhibit sex differences. In this work, we used single-nuclei transcriptomics to dissect the contributions of sex chromosomes and gonads in demyelination and AD. In a mouse model of demyelination, we identified the roles of sex chromosomes and gonads in modifying microglia and oligodendrocyte responses before and after myelin loss. In an AD-related mouse model expressing APOE4, XY sex chromosomes heightened interferon (IFN) response and tau-induced demyelination. The X-linked gene, Toll-like receptor 7 (Tlr7), regulated sex-specific IFN response to myelin. Deletion of Tlr7 dampened sex differences while protecting against demyelination. Administering TLR7 inhibitor mitigated tau-induced motor impairment and demyelination in male mice, indicating that Tlr7 plays a role in the male-biased type I Interferon IFN response in aging- and AD-related demyelination.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

