YAP1 preserves tubular mitochondrial quality control to mitigate diabetic kidney disease
- PMID: 39608245
- PMCID: PMC11629574
- DOI: 10.1016/j.redox.2024.103435
YAP1 preserves tubular mitochondrial quality control to mitigate diabetic kidney disease
Abstract
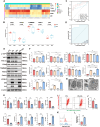
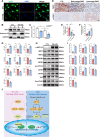
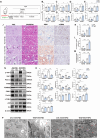
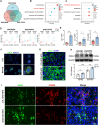
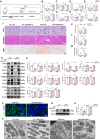
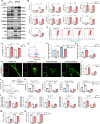
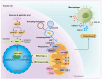
Renal tubule cells act as a primary site of injury in diabetic kidney disease (DKD), with dysfunctional mitochondrial quality control (MQC) closely associated with progressive kidney dysfunction in this context. Our investigation delves into the observed inactivation of yes-associated protein 1 (YAP1) and consequential dysregulation of MQC within renal tubule cells among DKD subjects through bioinformatic analysis of transcriptomics data from the Gene Expression Omnibus (GEO) dataset. Receiver operating characteristic curve analysis unequivocally underscores the robust diagnostic accuracy of YAP1 and MQC-related genes for DKD. Furthermore, we observed YAP1 inactivation, accompanied by perturbed MQC, within cultured tubule cells exposed to high glucose (HG) and palmitic acid (PA). This pattern was also evident in the tubulointerstitial compartment of kidney sections from biopsy-approved DKD patients. Additionally, renal tubule cell-specific Yap1 deletion exacerbated kidney injury in diabetic mice. Mechanistically, Yap1 deletion disrupted MQC, leading to mitochondrial aberrations in mitobiogenesis and mitophagy within tubule cells, ultimately culminating in histologic tubular injury. Notably, Yap1 deletion-induced renal tubule injury promoted the secretion of C-X-C motif chemokine ligand 1 (CXCL1), potentially augmenting M1 macrophage infiltration within the renal microenvironment. These multifaceted events were significantly ameliorated by administrating the YAP1 activator XMU-MP-1 in DKD mice. Consistently, bioinformatic analysis of transcriptomics data from the GEO dataset revealed a noteworthy upregulation of tubule cells-derived chemokine CXCL1 associated with macrophage infiltration among DKD patients. Crucially, overexpression of YAP1 via adenovirus transfection sustained mitochondrial membrane potential, mtDNA copy number, oxygen consumption rate, and activity of mitochondrial respiratory chain complex, but attenuated mitochondrial ROS production, thereby maintaining MQC and subsequently suppressing CXCL1 generation within cultured tubule cells exposed to HG and PA. Collectively, our study establishes a pivotal role of tubule YAP1 inactivation-mediated MQC dysfunction in driving DKD progression, at least in part, facilitated by promoting M1 macrophage polarization through a paracrine-dependent mechanism.
Keywords: Chemokine; Diabetic kidney disease; Hippo signaling pathway; Macrophage; Mitochondria; Yes-associated protein 1.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Tuttle K.R., Agarwal R., Alpers C.E., et al. Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int. 2022;102:248–260. - PubMed
-
- Liu B.C., Tang T.T., Lv L.L., et al. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int. 2018;93:568–579. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

