Identifying predictors of treatment response and molecular changes induced by neoadjuvant chemotherapy and endocrine therapy in hormone receptor-positive/HER2-negative breast cancer: the NEOENDO translational study
- PMID: 39608304
- PMCID: PMC11635665
- DOI: 10.1016/j.esmoop.2024.103989
Identifying predictors of treatment response and molecular changes induced by neoadjuvant chemotherapy and endocrine therapy in hormone receptor-positive/HER2-negative breast cancer: the NEOENDO translational study
Abstract
Background: Predictors of response to neoadjuvant chemotherapy (NACT) and endocrine therapy (NET) in hormone receptor-positive (HoR+)/human epidermal growth factor receptor 2 (HER2)-negative breast cancer (BC) are required. Also, pathological and molecular changes induced by both strategies and their impact on patients' outcomes have not been reported so far.
Patients and methods: In a cohort of 186 patients with early-stage HoR+/HER2-negative BC treated with NACT or NET, we assessed the association of baseline main clinicopathological features and PAM50 gene expression (GE), intrinsic subtypes (IS) and risk-of-relapse (ROR-P) score with pathological outcomes according to treatment strategy. Molecular NACT/NET-induced changes were described and compared, along with their associations with event-free survival (EFS). Comparison of the two cohorts after propensity score matching (PSM) was used as sensitivity analysis. Molecular changes were confirmed in cell lines.
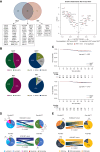
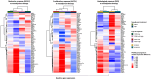
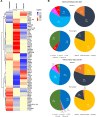
Results: NACT was associated with higher rates of residual cancer burden (RCB)-0/I than NET in the overall population (38.2% versus 13.5%, P < 0.001) and after PSM (P = 0.036). PAM50 non-luminal IS were the only independent and positive predictor of RCB-0/I (P = 0.024) in the NACT cohort, while MMP11 messenger RNA levels were the only independent and negative predictor (P = 0.014) in the NET cohort. Both treatments shifted the tumor types toward less aggressive forms (i.e. PAM50 luminal A/normal-like), lowered the risk of recurrence in terms of ROR-P, up-regulated selected immune genes and PAM50 basal-like-related genes/signature and significantly downregulated proliferation-/luminal-/HER2-related genes/signatures, though NACT more than NET. Molecular findings were confirmed after PSM. A net reduction in proliferation-related genes and ROR-P was confirmed in cell lines with chemotherapy and endocrine therapy. Different baseline molecular features associated with diverse kind of responses (ROR-P downstaging, Ki67 reduction or pathological responses) with NACT and NET. Decreasing ROR-P and transitioning the tumor subtype to resemble normal tissue (i.e. PAM50 normal-like) suggested improved EFS.
Conclusions: NACT was more effective in the molecular and dimensional tumor 'downstaging' than NET but baseline molecular features associated with differential responses according to treatment strategy. Examining baseline and post-treatment GE might help tailor more personalized and effective care.
Keywords: PAM50; breast cancer; intrinsic subtypes; molecular downstaging; neoadjuvant chemotherapy; neoadjuvant endocrine therapy.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Loibl S., André F., Bachelot T., et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2024;35(2):159–182. - PubMed
-
- Sirico M., Virga A., Conte B., et al. Neoadjuvant endocrine therapy for luminal breast tumors: state of the art, challenges and future perspectives. Crit Rev Oncol Hematol. 2023;181 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

