Maintenance with 5-FU/LV-aflibercept after induction with FOLFIRI-aflibercept versus FOLFIRI-aflibercept until progression as second-line treatment in older adults with metastatic colorectal cancer: the AFEMA phase II randomized trial
- PMID: 39608305
- PMCID: PMC11635659
- DOI: 10.1016/j.esmoop.2024.103986
Maintenance with 5-FU/LV-aflibercept after induction with FOLFIRI-aflibercept versus FOLFIRI-aflibercept until progression as second-line treatment in older adults with metastatic colorectal cancer: the AFEMA phase II randomized trial
Abstract
Background: The combination chemotherapy i.v. 5-fluorouracil (5-FU), irinotecan, and aflibercept (FOLFIRI-A) is a standard second-line treatment of metastatic colorectal cancer (mCRC). The aim was to assess maintenance treatment in second-line setting in older patients (aged ≥70 years) with mCRC.
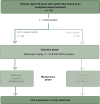
Patients and methods: We evaluated FOLFIRI-A given for six cycles followed by maintenance with 5-FU/leucovorin (LV)-A (arm A) or FOLFIRI-A (arm B) until progression in older adults with mCRC in the AFEMA randomized, open-label, non-inferiority phase II trial (EudraCT2016-004076-21/NCT03279289). Patients aged ≥70 years who previously failed oxaliplatin-fluoropyrimidine were randomly allocated (1 : 1) to either arm A (experimental) or arm B (control). After enrolling 35 patients, the FOLFIRI dose was reduced to level 1 in both arms due to toxicity. The primary endpoint was median progression-free survival (PFS); and secondary endpoints were median overall survival, objective response rate, and safety. Non-inferiority required the upper confidence interval (CI) limit to not exceed a hazard ratio (HR) of 1.5 (one-sided α = 0.075, 80% power).
Results: A total of 170 patients were randomly allocated to arm A or arm B (n = 85 each). The median follow-up was 12.2 versus 10.9 months in arm A versus arm B. Most patients died (83.5% versus 88.2% in arm A versus arm B), mainly from disease progression. PFS non-inferiority was met (HR = 0.78, 95% CI 0.566-1.076, P = 0.131) with a median PFS of 6.1 versus 5.5 months in arm A versus arm B. Median overall survival was similar in arms A and B (12.2 and 11.5 months, respectively) (HR = 0.89, 95% CI 0.640-1.227, P = 0.467). During the maintenance phase, severe asthenia (4.5% versus 21.6%, P = 0.038), serious adverse events (SAEs) (17.8% versus 37.8%, P = 0.049), and treatment-related SAEs (6.7% versus 10.8%, P = 0.695) were reduced in arm A versus arm B.
Conclusion: In older adults, induction with six cycles of FOLFIRI-A plus maintenance with 5-FU/LV-A was non-inferior to FOLFIRI-A until progression. Severe asthenia, SAEs, and treatment-related SAEs were reduced with 5-FU/LV-A maintenance.
Keywords: 5-FU; FOLFIRI; aflibercept; maintenance treatment; metastatic colorectal cancer.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Siegel R.L., Miller K.D., Goding Sauer A., et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145–164. - PubMed
-
- Biller L.H., Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325(7):669–685. - PubMed
-
- European Cancer Information System. https://ecis.jrc.ec.europa.eu/factsheets.php Available at.
-
- Dekker E., Tanis P.J., Vleugels J.L.A., Kasi P.M., Wallace M.B. Colorectal cancer. Lancet. 2019;394(10207):1467–1480. - PubMed
-
- Van Cutsem E., Cervantes A., Adam R., et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

