Molecular determinants of the selectivity and potency of α-conotoxin Vc1.1 for human nicotinic acetylcholine receptors
- PMID: 39608712
- PMCID: PMC11732461
- DOI: 10.1016/j.jbc.2024.108017
Molecular determinants of the selectivity and potency of α-conotoxin Vc1.1 for human nicotinic acetylcholine receptors
Abstract
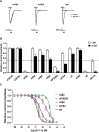
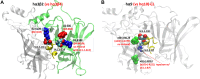
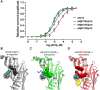
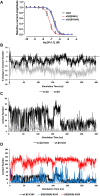
The α-conotoxins (α-Ctxs) are short, disulfide-rich peptides derived from the venom of the Conus marine snails, primarily acting as antagonists of nicotinic acetylcholine receptors (nAChRs). Specifically, α-Ctx Vc1.1, a 16-amino acid peptide from Conus victoriae, competitively antagonizes non-muscle nAChRs, inhibits nicotine-induced currents in bovine chromaffin cells, and alleviates neuropathic pain in rat models. Although Vc1.1 selectively inhibits rat α9α10 nAChRs, its potency and selectivity across human nAChR subtypes remain unresolved. In this study, we assessed the activity of Vc1.1 on human (h) nAChRs heterologously expressed in Xenopus laevis oocytes using the two-electrode voltage clamp technique and simulated interactions using computational modeling. Vc1.1 selectively antagonized homomeric α9 and heteromeric α3β2 nAChRs, with half-maximal inhibitory concentrations (IC50) of 160 nM and 232 nM, respectively. At hα9[N179A]α10, Vc1.1 exhibited a 20-fold decrease in potency compared to hα9α10, due to the loss of hydrogen bonding with Vc1.1-D11. Conversely, Vc1.1 was four-fold more potent at hα3β2[E86A] compared to hα3β2, possibly influenced by the proximal residue β2-K104, as suggested by molecular dynamics (MD) simulations. Additionally, Vc1.1's potency doubled at hα9[N213K]α10, whereas it remained unchanged at hα9[N213R]α10 nAChRs. MD simulations indicate that altered interactions between the mutant hα9 N179A, N213K, and N213R side chains and Vc1.1-D5 may partly explain these changes in potency. The inhibitory action of Vc1.1 at α9-containing nAChRs is particularly relevant given their role in neuroinflammation, presenting a potential therapeutic pathway for alleviating neuropathic and inflammatory pain. This study provides valuable insights into the rational design of Vc1.1-derived α-Ctxs with enhanced nAChR subtype selectivity.
Keywords: Xenopus oocytes; electrophysiology; molecular dynamics; nicotinic acetylcholine receptors (nAChR); site-directed mutagenesis; α-conotoxin.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflicts of interest with the contents of this article.
Figures
Similar articles
-
Scanning mutagenesis of alpha-conotoxin Vc1.1 reveals residues crucial for activity at the alpha9alpha10 nicotinic acetylcholine receptor.J Biol Chem. 2009 Jul 24;284(30):20275-84. doi: 10.1074/jbc.M109.015339. Epub 2009 May 15. J Biol Chem. 2009. PMID: 19447885 Free PMC article.
-
Globular and ribbon isomers of Conus geographus α-conotoxins antagonize human nicotinic acetylcholine receptors.Biochem Pharmacol. 2021 Aug;190:114638. doi: 10.1016/j.bcp.2021.114638. Epub 2021 May 29. Biochem Pharmacol. 2021. PMID: 34062129
-
A novel α4/7-conotoxin LvIA from Conus lividus that selectively blocks α3β2 vs. α6/α3β2β3 nicotinic acetylcholine receptors.FASEB J. 2014 Apr;28(4):1842-53. doi: 10.1096/fj.13-244103. Epub 2014 Jan 7. FASEB J. 2014. PMID: 24398291 Free PMC article.
-
Conotoxin Interactions with α9α10-nAChRs: Is the α9α10-Nicotinic Acetylcholine Receptor an Important Therapeutic Target for Pain Management?Toxins (Basel). 2015 Sep 28;7(10):3916-32. doi: 10.3390/toxins7103916. Toxins (Basel). 2015. PMID: 26426047 Free PMC article. Review.
-
The Structural Features of α-Conotoxin Specifically Target Different Isoforms of Nicotinic Acetylcholine Receptors.Curr Top Med Chem. 2015;16(2):156-69. doi: 10.2174/1568026615666150701114831. Curr Top Med Chem. 2015. PMID: 26126912 Review.
References
-
- Dani J.A., Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 2007;47:699–729. - PubMed
-
- Hurst R., Rollema H., Bertrand D. Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol. Ther. 2013;137:22–54. - PubMed
-
- Improgo M.R., Tapper A.R., Gardner P.D. Nicotinic acetylcholine receptor-mediated mechanisms in lung cancer. Biochem. Pharmacol. 2011;82:1015–1021. - PubMed
-
- Mucchietto V., Crespi A., Fasoli F., Clementi F., Gotti C. Neuronal acetylcholine nicotinic receptors as new targets for lung cancer treatment. Curr. Pharm. Des. 2016;22:2160–2169. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

