A conserved acidic residue drives thyroxine synthesis within thyroglobulin and other protein precursors
- PMID: 39608720
- PMCID: PMC11730217
- DOI: 10.1016/j.jbc.2024.108026
A conserved acidic residue drives thyroxine synthesis within thyroglobulin and other protein precursors
Abstract
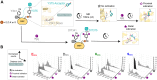
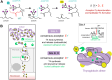
Thyroxine, the main hormone product of the thyroid, is produced at multiple sites within its protein precursor thyroglobulin. Each site consists of two tyrosine residues which undergo iodination and coupling, resulting in the synthesis of thyroxine at the acceptor tyrosine, where the hormone synthesis is later completed by proteolysis. Within the structurally resolved sites, the role of an essential conserved acidic residue preceding the acceptor remains elusive. To elucidate the mechanism of thyroxine synthesis we engineered a single-site minimal protein precursor. First, by its in vitro iodination and site-directed mutagenesis we show that the presence of the acidic residue, preferably glutamate, favors thyroxine synthesis. Secondly, within the designed precursor, we computationally modeled the reaction of iodination and iodotyrosine coupling giving rise to thyroxine. Our results reveal that hormone formation is triggered by iodotyrosine deprotonation, facilitated by proximity to a carboxylic group, closer in the case of glutamate, in line with our experimental findings and sequence conservation. Hereafter, we surmise that in the natural precursor thyroglobulin, two evolutionary late and slower hormonogenic sites coexist with an early evolutionary and faster one. Indeed, the latter is overlapping with a proteolytic site, thereby allowing prompt thyroxine release from thyroglobulin.
Keywords: density functional theory; iodination; molecular dynamics; protein engineering; thyroglobulin; thyroid hormones; thyroxine.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


Similar articles
-
Hormone formation in the isolated fragment 1-171 of human thyroglobulin involves the couple tyrosine 5 and tyrosine 130.Mol Cell Endocrinol. 1991 Oct;81(1-3):155-64. doi: 10.1016/0303-7207(91)90214-d. Mol Cell Endocrinol. 1991. PMID: 1686772
-
Kinetics of thyroglobulin iodination and of hormone synthesis catalysed by thyroid peroxidase. Role of iodide in the coupling reaction.Eur J Biochem. 1976 Nov 15;70(2):435-40. doi: 10.1111/j.1432-1033.1976.tb11034.x. Eur J Biochem. 1976. PMID: 1009939
-
Characterization of hormonogenic sites in an N-terminal, cyanogen bromide fragment of human thyroglobulin.Arch Biochem Biophys. 1995 Jun 20;320(1):96-105. doi: 10.1006/abbi.1995.1346. Arch Biochem Biophys. 1995. PMID: 7793989
-
The role of thyroglobulin in thyroid hormonogenesis.Nat Rev Endocrinol. 2019 Jun;15(6):323-338. doi: 10.1038/s41574-019-0184-8. Nat Rev Endocrinol. 2019. PMID: 30886364 Review.
-
The importance of thyroglobulin structure for thyroid hormone biosynthesis.Biochimie. 1999 May;81(5):505-9. doi: 10.1016/s0300-9084(99)80102-3. Biochimie. 1999. PMID: 10403182 Review.
Cited by
-
Metabolic Pattern of Brain Death-NMR-Based Metabolomics of Cerebrospinal Fluid.Int J Mol Sci. 2025 Mar 18;26(6):2719. doi: 10.3390/ijms26062719. Int J Mol Sci. 2025. PMID: 40141360 Free PMC article.

