Efficacy and Safety of Subcutaneous Abatacept Plus Standard Treatment for Active Idiopathic Inflammatory Myopathy: Phase 3 Randomized Controlled Trial
- PMID: 39609094
- PMCID: PMC12123255
- DOI: 10.1002/art.43066
Efficacy and Safety of Subcutaneous Abatacept Plus Standard Treatment for Active Idiopathic Inflammatory Myopathy: Phase 3 Randomized Controlled Trial
Abstract
Objective: Our objective was to evaluate the efficacy and safety of subcutaneous (SC) abatacept and standard of care (SOC) for the treatment of idiopathic inflammatory myopathy (IIM) over 52 weeks.
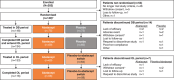
Methods: In this randomized, double-blind, placebo-controlled phase III trial, patients with treatment-refractory IIM received SC abatacept (at 125 mg weekly) with SOC (abatacept group) or a placebo with SOC (placebo group). A 24-week double-blind period was followed by an open-label period to assess outcomes from continued therapy with abatacept and initiation with abatacept (placebo-to-abatacept switch group) from 24 to 52 weeks. The primary end point was International Myositis Assessment and Clinical Studies definition of improvement (IMACS DOI) at week 24. Secondary efficacy and safety end points were assessed.
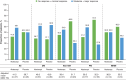
Results: Overall, 148 (double-blind) and 133 (open-label) patients were treated. Baseline demographics were well-balanced between treatment groups and disease subtypes. At 24 weeks, improvement per IMACS DOI was 56.0% for the abatacept group and 42.5% for the placebo group (P = 0.083); at 52 weeks, improvement was 69.8% (continued abatacept) and 69.0% (placebo-to-abatacept switch). The IMACS DOI rate at 24 weeks was greater in the nondermatomyositis (non-DM) group (abatacept: 57.1%; placebo: 32.3%; P = 0.040) than the DM group (abatacept: 55.0%; placebo: 50.0%; P = 0.679). The observed safety profile was similar in both groups.
Conclusion: The proportion of patients who met improvement criteria after 24 weeks was similar between abatacept and placebo groups. However, analysis by IIM subtype suggested there may be a sustained benefit of SC abatacept for patients with non-DM subtypes.
© 2024 The Author(s). Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures
References
-
- Miller FW. Classification of idiopathic inflammatory myopathies. In: Kagen L, ed. The Inflammatory Myopathies. Humana Press; 2009.
-
- Lundberg IE, Tjärnlund A, Bottai M, et al; International Myositis Classification Criteria Project Consortium, the Euromyositis Register, and the Juvenile Dermatomyositis Cohort Biomarker Study and Repository (UK and Ireland) . 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Arthritis Rheumatol 2017;69(12):2271–2282. - PMC - PubMed
-
- Iaccarino L, Gatto M, Bettio S, et al. Overlap connective tissue disease syndromes. Autoimmun Rev 2013;12(3):363–373. - PubMed
-
- Baer AN. Differential diagnosis of idiopathic inflammatory myopathies. Curr Rheumatol Rep 2006;8(3):178–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

