Bacteria-targeted imaging using vancomycin-based positron emission tomography tracers can distinguish infection from sterile inflammation
- PMID: 39609275
- PMCID: PMC11928434
- DOI: 10.1007/s00259-024-06997-z
Bacteria-targeted imaging using vancomycin-based positron emission tomography tracers can distinguish infection from sterile inflammation
Abstract
Introduction: Bacterial infections pose major challenges in medicine. To guide effective infection treatment, faster and more accurate diagnostic modalities are needed. Bacteria-targeted molecular imaging can meet these needs. The present study was aimed at the in vivo evaluation of two 18F-vancomycin-based PET tracers, for detection of deep-seated Gram-positive bacterial infections. These tracers were bench-marked against the current standard of care, [18F]FDG.
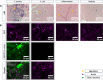
Methods: The potential of [18F]BODIPY-FL-vancomycin and [18F]PQ-VE1-vancomycin ([4+2]photocycloadduct of 9,10-phenanthrenequinone-vancomycin and [18F]fluorinated vinyl ether) to distinguish bacterial infections from sterile inflammation was evaluated in a murine myositis model. Tracer specificity was assessed by infecting mice either with the Gram-positive bacterium Staphylococcus aureus (n = 12) or the Gram-negative bacterium Escherichia coli (n = 12). The contralateral leg was injected with Cytodex beads to induce sterile inflammation, or with phosphate-buffered saline for control. In parallel, mice were imaged with [18F]FDG (n = 12). Dynamic positron emission tomography (PET) measurements, biodistribution analyses, and immunohistopathology were performed to determine tracer distribution and bacterial burden.
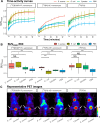
Results: Both 18F-vancomycin-PET tracers accumulated at sites of infection, but not at sites of sterile inflammation, in contrast to [18F]FDG. The tracers exhibited distinct biodistribution profiles, with [18F]BODIPY-FL-vancomycin being cleared more rapidly. Both 18F-vancomycin-PET tracers displayed significant target to non-target ratios of 2.95 for [18F]BODIPY-FL-vancomycin and 1.48 for [18F]PQ-VE1-vancomycin.
Conclusion: Vancomycin-based PET is a potentially attractive approach to distinguish Gram-positive bacterial infections from sterile inflammation.
Keywords: 18F-vancomycin; Antibiotic-based tracer; Bacterial infection imaging; In vivo; Myositis; PET; Vancomycin.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: All experiments were conducted in accordance with Dutch and EU law and were performed in the Central Animal Facility of the University of Groningen (Centrale Dienst Dierproeven). Experimental protocols were approved by the National Committee on Animal Experiments of The Netherlands (Centrale Commissie Dierproeven, Den Haag) and the Institutional Animal Care and Use Committee of the University of Groningen (Instantie voor Dierproeven (IvD), CCD license number: AVD10500202114768. IvD licence number: 2114768-01-003). Competing interests: JMvD has filed a patent application on the use of 1D9, which is owned by his employer University Medical Center Groningen. The other authors declare no conflicts of interest.
Figures


Similar articles
-
Synthesis and preclinical evaluation of novel 18F-vancomycin-based tracers for the detection of bacterial infections using positron emission tomography.Eur J Nucl Med Mol Imaging. 2024 Jul;51(9):2583-2596. doi: 10.1007/s00259-024-06717-7. Epub 2024 Apr 22. Eur J Nucl Med Mol Imaging. 2024. PMID: 38644432 Free PMC article.
-
Efficacy of vancomycin-releasing biodegradable poly(lactide-co-glycolide) antibiotics beads for treatment of experimental bone infection due to Staphylococcus aureus.J Orthop Surg Res. 2016 Apr 27;11(1):52. doi: 10.1186/s13018-016-0386-x. J Orthop Surg Res. 2016. PMID: 27121956 Free PMC article.
-
d-[5-11C]-Glutamine Positron Emission Tomography Imaging for Diagnosis and Therapeutic Monitoring of Orthopedic Implant Infections.ACS Infect Dis. 2025 Jan 10;11(1):144-154. doi: 10.1021/acsinfecdis.4c00487. Epub 2024 Oct 15. ACS Infect Dis. 2025. PMID: 39410659
-
Imaging of infection and inflammation with 18F-FDG-labeled leukocytes.Q J Nucl Med Mol Imaging. 2006 Jun;50(2):143-6. Q J Nucl Med Mol Imaging. 2006. PMID: 16770304 Review.
-
Proliferation markers for the differential diagnosis of tumor and inflammation.Curr Pharm Des. 2008;14(31):3326-339. doi: 10.2174/138161208786549399. Curr Pharm Des. 2008. PMID: 19075707 Review.
Cited by
-
Vancomycin-based tracers guiding in situ visualization of bacteria on osteosynthesis devices and surgical debridement.Eur J Nucl Med Mol Imaging. 2025 Aug;52(10):3877-3890. doi: 10.1007/s00259-025-07249-4. Epub 2025 Apr 2. Eur J Nucl Med Mol Imaging. 2025. PMID: 40172692 Free PMC article.
-
Diagnostic Value of Increased [18F]FDG Uptake in Locoregional Lymph Nodes on PET/CT in Patients with Suspected Fracture-Related Infection.Diagnostics (Basel). 2025 Mar 4;15(5):616. doi: 10.3390/diagnostics15050616. Diagnostics (Basel). 2025. PMID: 40075863 Free PMC article.
References
-
- O’Neill J. Review on antimicrobial resistance: tackling drug-resistant infections globally: final report and recommendations. Wellcome Trust; 2016. p. 80.
-
- Glaudemans AW, et al. Diagnosing fracture-related infections: can we optimize our nuclear imaging techniques? Eur J Nucl Med Mol Imaging. 2019;46(8):1583–7. - PubMed
-
- van Oosten M, et al. Real-time in vivo imaging of invasive- and biomaterial-associated bacterial infections using fluorescently labelled vancomycin. Nat Commun. 2013;4(1):2584. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

