Nucleic acid drugs: recent progress and future perspectives
- PMID: 39609384
- PMCID: PMC11604671
- DOI: 10.1038/s41392-024-02035-4
Nucleic acid drugs: recent progress and future perspectives
Abstract
High efficacy, selectivity and cellular targeting of therapeutic agents has been an active area of investigation for decades. Currently, most clinically approved therapeutics are small molecules or protein/antibody biologics. Targeted action of small molecule drugs remains a challenge in medicine. In addition, many diseases are considered 'undruggable' using standard biomacromolecules. Many of these challenges however, can be addressed using nucleic therapeutics. Nucleic acid drugs (NADs) are a new generation of gene-editing modalities characterized by their high efficiency and rapid development, which have become an active research topic in new drug development field. However, many factors, including their low stability, short half-life, high immunogenicity, tissue targeting, cellular uptake, and endosomal escape, hamper the delivery and clinical application of NADs. Scientists have used chemical modification techniques to improve the physicochemical properties of NADs. In contrast, modified NADs typically require carriers to enter target cells and reach specific intracellular locations. Multiple delivery approaches have been developed to effectively improve intracellular delivery and the in vivo bioavailability of NADs. Several NADs have entered the clinical trial recently, and some have been approved for therapeutic use in different fields. This review summarizes NADs development and evolution and introduces NADs classifications and general delivery strategies, highlighting their success in clinical applications. Additionally, this review discusses the limitations and potential future applications of NADs as gene therapy candidates.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
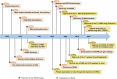


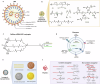
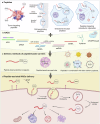

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

