Mechanistic basis for the emergence of EPS1 as a catalyst in salicylic acid biosynthesis of Brassicaceae
- PMID: 39609394
- PMCID: PMC11605079
- DOI: 10.1038/s41467-024-54437-1
Mechanistic basis for the emergence of EPS1 as a catalyst in salicylic acid biosynthesis of Brassicaceae
Abstract
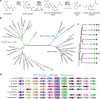
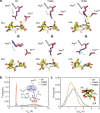
Salicylic acid (SA) production in Brassicaceae plants is uniquely accelerated from isochorismate by EPS1, a newly identified enzyme in the BAHD acyltransferase family. We present crystal structures of EPS1 from Arabidopsis thaliana in both its apo and substrate-analog-bound forms. Integrating microsecond-scale molecular dynamics simulations with quantum mechanical cluster modeling, we propose a pericyclic rearrangement lyase mechanism for EPS1. We further reconstitute the isochorismate-derived SA biosynthesis pathway in Saccharomyces cerevisiae, establishing an in vivo platform to examine the impact of active-site residues on EPS1 functionality. Moreover, stable transgenic expression of EPS1 in soybean increases basal SA levels, highlighting the enzyme's potential to enhance defense mechanisms in non-Brassicaceae plants lacking an EPS1 ortholog. Our findings illustrate the evolutionary adaptation of an ancestral enzyme's active site to enable a novel catalytic mechanism that boosts SA production in Brassicaceae plants.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: J.K.W. is a member of the Scientific Advisory Board and a shareholder of DoubleRainbow Biosciences, Galixir, and Inari Agriculture, which develop biotechnologies related to natural products, drug discovery, and agriculture. All other authors have no competing interests.
Figures




References
-
- Raskin, I. Role of salicylic acid in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol.43, 439–463 (1992). - DOI
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

