Outcomes with loncastuximab tesirine following CAR T-cell therapy in patients with relapsed or refractory diffuse large B-cell lymphoma
- PMID: 39609431
- PMCID: PMC11604956
- DOI: 10.1038/s41408-024-01195-4
Outcomes with loncastuximab tesirine following CAR T-cell therapy in patients with relapsed or refractory diffuse large B-cell lymphoma
Abstract
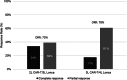
The efficacy of loncastuximab tesirine (lonca) following chimeric antigen receptor T-cell therapy (CAR-T) progression/failure is unknown. Hence, we sought to examine real-world use and outcomes of lonca following CAR-T in patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) in the USA. In this retrospective study, we included adults (age ≥ 18 years) with R/R DLBCL who received lonca monotherapy as third- (3 L) or fourth line (4 L) treatment after progressing on second line (2 L) or 3 L CAR-T, respectively. Post-CAR-T lonca outcomes included response rates (overall response rate [ORR] and complete response [CR] rate), duration of response (DOR), progression-free survival (PFS), and overall survival (OS). A total of 118 patients were included in the analysis with 95 receiving lonca following 2 L CAR-T (median age:66 years; 61% male) and 23 following 3 L CAR-T (median age:57 years; 43% male). Patients with 2 L CAR-T/3 L lonca had an ORR of 73% (CR rate of 34%). With a median follow-up of 8.5 months following lonca initiation, median DOR, PFS, and OS were not reached. The DOR, PFS, and OS at 12 months were 68%, 77%, and 84%, respectively. Patients with 3 L CAR-T/4 L lonca had an ORR of 78% (CR rate of 17%). With a median follow-up of 13 months following lonca initiation, the median DOR and PFS were 7.6 and 12.0 months, while median OS was not reached. OS at 12 months was 95%. In this study, we found that lonca monotherapy was an effective treatment option in R/R DLBCL in 3 L and 4 L settings including those who were resistant to or progressed after CAR-T.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: NE provides consultancy for ADC Therapeutics. He is a member of the Speakers Bureau for Beigene, and Genentech. He also received research funding from Beigene, Lilly, and Incyte. TB, LM, JC, MA and GM are employees of Adelphi Real World, who received funding from ADC Therapeutics for this work. ML and LC were employees of ADC Therapeutics at the time of the study and currently own stock in the company.
Figures
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. - PubMed
-
- Al-Hamadani M, Habermann TM, Cerhan JR, Macon WR, Maurer MJ, Go RS. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: a longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol. 2015;90:790–5. - PubMed
-
- Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources