Microglia regulate motor neuron plasticity via reciprocal fractalkine and adenosine signaling
- PMID: 39609435
- PMCID: PMC11605081
- DOI: 10.1038/s41467-024-54619-x
Microglia regulate motor neuron plasticity via reciprocal fractalkine and adenosine signaling
Abstract
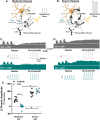
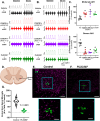
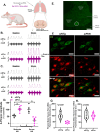
We report an important role for microglia in regulating neuroplasticity within phrenic motor neurons. Brief episodes of low oxygen (acute intermittent hypoxia; AIH) elicit a form of respiratory motor plasticity known as phrenic long-term facilitation (pLTF) that is regulated by the balance of competing serotonin vs adenosine-initiated cellular mechanisms. Serotonin arises from brainstem raphe neurons, but the source of adenosine is unknown. We tested if hypoxic episodes initiate phrenic motor neuron to microglia fractalkine signaling that evokes extracellular adenosine formation using a well-defined neurophysiology preparation in male rats. With moderate AIH, phrenic motor neuron adenosine 2A receptor activation undermines serotonin-dominant pLTF whereas severe AIH induces pLTF by the adenosine-dependent mechanism. Consequently, phrenic motor neuron fractalkine knockdown, microglial fractalkine receptor inhibition, and microglial ablation enhance moderate AIH, but suppress severe AIH-induced pLTF. We conclude, microglia play important roles in healthy spinal cords, regulating plasticity in motor neurons responsible for breathing.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



Update of
-
Microglia regulate motor neuron plasticity via reciprocal fractalkine/adenosine signaling.bioRxiv [Preprint]. 2024 May 9:2024.05.07.592939. doi: 10.1101/2024.05.07.592939. bioRxiv. 2024. Update in: Nat Commun. 2024 Nov 28;15(1):10349. doi: 10.1038/s41467-024-54619-x. PMID: 38765982 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01HL149800/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- T32 HL134621/HL/NHLBI NIH HHS/United States
- R01 HL149800/HL/NHLBI NIH HHS/United States
- T32HL134621-5/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01HL148030/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
LinkOut - more resources
Full Text Sources
Research Materials

