Adjuvants to the S1-subunit of the SARS-CoV-2 spike protein vaccine improve antibody and T cell responses and surrogate neutralization in mice
- PMID: 39609527
- PMCID: PMC11604653
- DOI: 10.1038/s41598-024-80636-3
Adjuvants to the S1-subunit of the SARS-CoV-2 spike protein vaccine improve antibody and T cell responses and surrogate neutralization in mice
Abstract
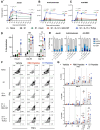
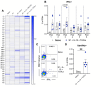
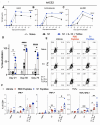
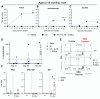
Various public health measures have contained outbreaks of SARS-CoV-2, but concerns remain over the possibility of future surges. Improvements in broadening the vaccine response can stifle new and nascent infections. In this study, we tested the effects of different adjuvant combinations on the immunization of mice with the receptor-binding domain (RBD)-containing the S1-subunit of the spike protein (S1 protein) from SARS-CoV-2 to induce a robust humoral and cellular immune response. We showed that subcutaneous immunization of S1 protein co-delivered with IL-15 and TLR-ligands (MALP-2, poly I: C, and CpG) or with IL-12 and GM-CSF in DOTAP, or Alum induced significantly high titers of durable antibodies, predominantly IgG1, IgG2a, and IgG2b, that could bind to RBD, S1-subunit, and the full-length ectodomain of SARS-CoV-2 spike protein in sera compared to the immunization with S1 protein alone in both B6 wild-type (WT) and the K18-hACE2 transgenic mice. In addition, immunization with S1 protein co-delivered with IL-15 and TLR-ligands induced antibody responses against S1 protein in aged mice, and sera from younger mice reduced plaque formation of live SARS-CoV-2, and had effective binding to S1 protein from ten different variants of SARS-CoV-2, including Omicron (B.1.1.529), and greater neutralization activity as early as day 21 post-immunization measured by inhibition of RBD binding to hACE2 than sera from mice immunized with S1 protein alone or co-delivered with Alum. We also identified antibody-binding epitopes using 18-mer peptides with 9-residue overlaps from the S1 protein. CD8+ T-cell responses specific to RBD and S1 protein peptide pools were observed up to day 200 post-immunization by tetramer staining. These data show the efficacy of specific immunologically targeted adjuvants for increasing S1 protein immunogenicity in mice and can contribute to more effective vaccines.
Keywords: Adjuvants; Aging; SARS-CoV-2; Vaccine; Variants.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

