Distinguish active tuberculosis with an immune-related signature and molecule subtypes: a multi-cohort analysis
- PMID: 39609541
- PMCID: PMC11605007
- DOI: 10.1038/s41598-024-80072-3
Distinguish active tuberculosis with an immune-related signature and molecule subtypes: a multi-cohort analysis
Abstract
Background: Distinguishing latent tuberculosis infection (LTBI) from active tuberculosis (ATB) is very important. This study aims to analyze cases from multiple cohorts and get the signature that can distinguish LTBI from ATB.
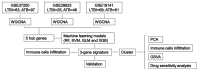
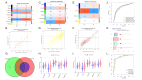
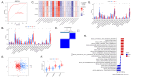
Methods: Thirteen datasets were downloaded from the gene expression omnibus (GEO) database. Three datasets were selected as discovery datasets, and the hub genes were discovered through WGCNA. In the training cohort, we use machine learning to establish the signature, verify the authentication ability of the signature in the remaining datasets, and compare it with other signatures. Cluster analysis was carried out on ATB cases, immune cell infiltration analysis, GSVA analysis, and drug sensitivity analysis were carried out on different clusters.
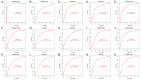
Results: In the discovery datasets, we discovered five hub genes. A signature (SLC26A8, ANKRD22, and FCGR1B) is obtained in the training cohort. In the total cohort, the three-gene signature can separate LTBI from ATB (the total area under ROC curve (AUC) is 0.801, 95% CI 0.771-0.830). Compared with other author's signatures, our signature shows good identification ability. Immunological analysis showed that SLC26A8, ANKRD22, and FCGR1B were closely related to the infiltration of immune cells. According to the expression of the three genes, ATB can be divided into two clusters, which are different in immune cell infiltration analysis, gene set variation, and drug sensitivity.
Conclusion: Our study produced an immune-related three-gene signature to distinguish LTBI from ATB, which may help us to manage and treat tuberculosis patients.
Keywords: Active tuberculosis; Immune infiltration; Latent tuberculosis infection; Molecule subtypes; Signature.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests. Ethical approval and consent to participate: Not applicable.
Figures

References
-
- In WHO consolidated guidelines on tuberculosis: Module 2: screening – systematic screening for tuberculosis disease (World Health Organization© World Health Organization 2021). (2021). - PubMed
-
- Organization, W. H. Global tuberculosis report 2023. Geneva: World Health Organ. (2023).
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

