A competing risk model analysis of dexmedetomidine of in-hospital mortality in subarachnoid hemorrhage patients
- PMID: 39609577
- PMCID: PMC11604967
- DOI: 10.1038/s41598-024-81025-6
A competing risk model analysis of dexmedetomidine of in-hospital mortality in subarachnoid hemorrhage patients
Abstract
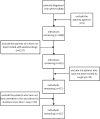
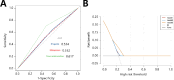
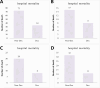
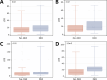
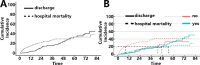
Subarachnoid hemorrhage (SAH) is a severe cerebrovascular disorder characterized by the sudden influx of blood into the subarachnoid space. The use of sedatives may be associated with the prognosis of SAH patients. We obtained SAH data from the MIMIC-IV database. The receiver operating characteristic curve, Delong test, and decision curve analysis were used to assess the predictive value of sedatives. Propensity score matching (PSM) method was applied to match samples at a 1:1 ratio. Logistic regression analysis, generalized linear regression analysis, and stratified analysis were used to investigate the association of the sedative with in-hospital mortality and length of hospital stay (LOS). Finally, a competing risk analysis was performed to evaluate the survival probability with two potential outcomes. Dexmedetomidine had a better prognosis value than Propofol and Midazolam. After PSM analysis, the Dexmedetomidine and the non-Dexmedetomidine groups had 248 samples each. The application of Dexmedetomidine reduced the risk of in-hospital mortality but might prolong the LOS. When considering in-hospital mortality as a competing risk factor for LOS, Dexmedetomidine was a protective factor for in-hospital mortality but had no significant relationship with LOS. In conclusion, treatment of Dexmedetomidine could reduce the risk of in-hospital mortality with satisfactory predictive efficiency.
Keywords: Competing risk analysis; Dexmedetomidine; In-hospital mortality; Length of hospital stay; Subarachnoid hemorrhage.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval: The Ethics Committee of Longyan First Hospital Affiliated to Fujian Medical University deemed that this research is based on open-source data, so the need for ethics approval was waived.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

