Prenatal dexamethasone exposure reduces osteoprogenitor proliferation in mice via histone modifications at the Mkp-1 gene locus
- PMID: 39609620
- PMCID: PMC11604782
- DOI: 10.1038/s42003-024-07288-x
Prenatal dexamethasone exposure reduces osteoprogenitor proliferation in mice via histone modifications at the Mkp-1 gene locus
Abstract
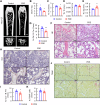
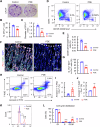
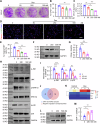
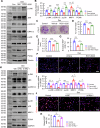
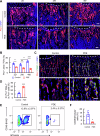
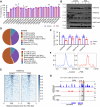
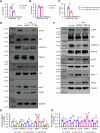
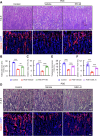
Prenatal dexamethasone exposure (PDE) has long-term consequences in bone development, though the underlying mechanisms remain unclear. Our results show that PDE offspring exhibit reduced bone mass, fewer osteoblasts and diminished osteoprogenitors proliferation. Further analyses show that PDE increases MKP-1 expression, while decreasing H3 lysine 9 dimethylation (H3K9me2) and H3 lysine 27 trimethylation (H3K27me3) at the Mkp-1 gene locus. Mechanistically, dexamethasone suppresses osteoprogenitors proliferation by upregulating MKP-1 expression, notably through the inhibition of H3K9me2 and H3K27me3 modifications, which promote demethylation and transcriptional activation of the Mkp-1 gene. Importantly, restoring histone methylation balance with PFI-90 or GSK-J4 treatment blocks the inhibitory effects of PDE on MAPK signaling in osteoprogenitors, and mitigates the detrimental impact of PDE on osteoprogenitor proliferation and bone development in the offspring. This study provides new insights into the epigenetic mechanism by which PDE disrupts long-term programming of fetal osteoprogenitor proliferation, ultimately impairing long bone growth in offspring.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval: All animal experiments were ethically approved by the Animal Ethics Committee of Southern Medical University Nanfang Hospital.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

