Closed and open structures of the eukaryotic magnesium channel Mrs2 reveal the auto-ligand-gating regulation mechanism
- PMID: 39609652
- PMCID: PMC11919701
- DOI: 10.1038/s41594-024-01432-1
Closed and open structures of the eukaryotic magnesium channel Mrs2 reveal the auto-ligand-gating regulation mechanism
Abstract
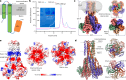
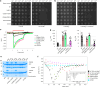
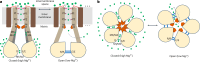
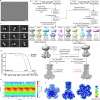
The CorA/Mrs2 family of pentameric proteins are cardinal for the influx of Mg2+ across cellular membranes, importing the cation to mitochondria in eukaryotes. Yet, the conducting and regulation mechanisms of permeation remain elusive, particularly for the eukaryotic Mrs2 members. Here, we report closed and open Mrs2 cryo-electron microscopy structures, accompanied by functional characterization. Mg2+ flux is permitted by a narrow pore, gated by methionine and arginine residues in the closed state. Transition between the conformations is orchestrated by two pairs of conserved sensor-serving Mg2+-binding sites in the mitochondrial matrix lumen, located in between monomers. At lower levels of Mg2+, these ions are stripped, permitting an alternative, symmetrical shape, maintained by the RDLR motif that replaces one of the sensor site pairs in the open conformation. Thus, our findings collectively establish the molecular basis for selective Mg2+ influx of Mrs2 and an auto-ligand-gating regulation mechanism.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures












References
-
- Maguire, M. E. & Cowan, J. A. Magnesium chemistry and biochemistry. Biometals15, 203–210 (2002). - PubMed
-
- Cowan, J. A. Structural and catalytic chemistry of magnesium-dependent enzymes. Biometals15, 225–235 (2002). - PubMed
-
- Hartwig, A. Role of magnesium in genomic stability. Mutat. Res475, 113–121 (2001). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

