Procalcitonin-guided antibiotic treatment in patients with cancer: a patient-level meta-analysis from randomized controlled trials
- PMID: 39609770
- PMCID: PMC11606202
- DOI: 10.1186/s12885-024-13160-2
Procalcitonin-guided antibiotic treatment in patients with cancer: a patient-level meta-analysis from randomized controlled trials
Abstract
Background: Use of serum procalcitonin (PCT), an inflammatory biomarker for bacterial infections, has shown promising results for early stopping antibiotic treatment among patients with respiratory infections and sepsis. There is need for additional data regarding effectiveness and safety of this concept among patients with cancer.
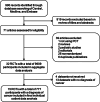
Methods: Individual data of patients with a documented diagnosis of cancer and proven or suspected respiratory infection and/or sepsis were extracted from previous trials where adult patients were randomized to receive antibiotic treatment based on a PCT protocol or usual care (control group). The primary efficacy and safety endpoints were antibiotic exposure and 28-day all-cause mortality.
Results: This individual-patient data meta-analysis included 777 patients with a diagnosis of cancer from 15 randomized-controlled trials. Regarding efficacy, there was a 18% reduction in antibiotic exposure in patients randomized to PCT-guided care compared to usual care ([days] 8.2 ± 6.6 vs. 9.8 ± 7.3; adjusted difference, - 1.77 [95% CI, - 2.74 to - 0.80]; p < 0.001). Regarding safety, there were 72 deaths in 379 patients in the PCT-guided group (19.0%) compared to 91 deaths in 398 participants in the usual care group (22.9%) resulting in an adjusted OR of 0.78 (95% CI, 0.60 to 1.02). A subgroup analysis showed a significant reduction in mortality in patients younger than 70 years (adjusted OR, 0.58 [95% CI, 0.40 to 0.86]).
Conclusion: Result of this individual patient meta-analysis from 15 previous trials suggests that among patients with cancer and suspected or proven respiratory infection or sepsis, use of PCT to guide antibiotic treatment decisions results in reduced antibiotic exposure with a possible reduction in mortality, particularly among younger patients.
Keywords: Antibiotic treatment; Cancer; Meta-analysis; Procalcitonin.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Human ethics and consent to participate: Not applicable due to the meta-analysis design. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

