Monitoring circulating tumor DNA liquid biopsy in stage III BRAF-mutant melanoma patients undergoing adjuvant treatment
- PMID: 39609824
- PMCID: PMC11603725
- DOI: 10.1186/s12967-024-05783-7
Monitoring circulating tumor DNA liquid biopsy in stage III BRAF-mutant melanoma patients undergoing adjuvant treatment
Abstract
Background: The introduction of adjuvant therapies for patients with resected cutaneous melanoma (CM) has increased the need for sensitive biomarkers for risk stratification and disease monitoring. This study aims to investigate the utility of circulating tumor DNA (ctDNA) assessment in predicting and reflecting disease status during adjuvant therapy.
Methods: We enrolled 32 patients with resected BRAF-mutated stage III CM receiving adjuvant targeted therapy or immunotherapy. Plasma samples of patients were collected at the baseline (treatment initiation) and during the therapy, and BRAF-mutated ctDNA was quantified by droplet digital PCR (ddPCR).
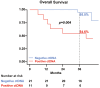
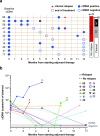
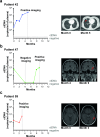
Results: Baseline ctDNA was detected in 11/32 (34.4%) patients and predicted postoperative high risk of relapse [HR 3.79, 95% CI 1.20-12.00, p = 0.023]. The three-year overall survival (OS) rate was 54.6% (95% CI 22.9-77.9) versus 95% (95% CI 69.5-99.3) in ctDNA-positive and negative groups, respectively, with significantly worse OS for ctDNA-positive patients [HR 7.92, 95% CI 1.56-40.36, p = 0.013]. Among the baseline ctDNA-positive group (high-risk patients), longitudinal ctDNA detection during adjuvant therapy reflected the clinical outcomes. Only non-relapsing patients cleared their plasma ctDNA by the end of the treatment, while persistent ctDNA detection provided early evidence of disease recurrence.
Conclusions: ctDNA detection shows promising results in the post-operative setting for identifying cutaneous melanoma patients at the highest risk of relapse and for real-time monitoring of patients' clinical status and treatment response.
Keywords: Adjuvant therapy; Cutaneous melanoma; Disease monitoring; Liquid biopsy; ddPCR.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by Comitato Etico Interaziendale AOU Città della Salute e della Scienza di Torino (TESEO − 0061280 - CE CS2/1306) and was conducted in accordance with the ethical standards of the University of Turin IRB and the principles of the declaration of Helsinki. Informed consent: Informed consent to surgical procedure and data collection was obtained from all subjects involved in the study. Competing interests: The authors declare no conflict of interest.
Figures

References
-
- Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer staging Manual: continuing to build a bridge from a population-based to a more personalized approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–9. - PubMed
-
- Ascierto PA, Del Vecchio M, Mandala M, Gogas H, Arance AM, Dalle S, et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(11):1465–77. - PubMed
-
- Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N Engl J Med. 2018;378(19):1789–801. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

