Genetic engineering of Nannochloropsis oceanica to produce canthaxanthin and ketocarotenoids
- PMID: 39609835
- PMCID: PMC11606307
- DOI: 10.1186/s12934-024-02599-4
Genetic engineering of Nannochloropsis oceanica to produce canthaxanthin and ketocarotenoids
Abstract
Background: Canthaxanthin is a ketocarotenoid with high antioxidant activity, and it is primarily produced by microalgae, among which Nannochloropsis oceanica, a marine alga widely used for aquaculture. In the last decade, N. oceanica has become a model organism for oleaginous microalgae to develop sustainable processes to produce biomolecules of interest by exploiting its photosynthetic activity and carbon assimilation properties. N. oceanica can accumulate lipids up to 70% of total dry weight and contains the omega-3 fatty acid eicosapentaenoic acid (EPA) required for both food and feed applications. The genome sequence, other omics data, and synthetic biology tools are available for this species, including an engineered strain called LP-tdTomato, which allows homologous recombination to insert the heterologous genes in a highly transcribed locus in the nucleolus region. Here, N. oceanica was engineered to induce high ketocarotenoid and canthaxanthin production.
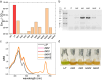
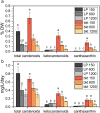
Results: We used N. oceanica LP-tdTomato strain as a background to express the key enzyme for ketocarotenoid production, a β-carotene ketolase (CrBKT) from Chlamydomonas reinhardtii. Through the LP-tdTomato strain, the transgene insertion by homologous recombination in a highly transcribed genomic locus can be screened by negative fluorescence. The overexpression of CrBKT in bkt transformants increased the content of carotenoids and ketocarotenoids per cell, respectively, 1.5 and 10-fold, inducing an orange/red color in the bkt cell cultures. Background (LP) and bkt lines productivity were compared at different light intensities from 150 to 1200 µmol m-2 s-1: at lower irradiances, the growth kinetics of bkt lines were slower compared to LP, while higher productivity was measured for bkt lines at 1200 µmol m-2 s-1. Despite these results, the highest canthaxanthin and ketocarotenoids productivity were obtained upon cultivation at 150 µmol m-2 s-1.
Conclusions: Through targeted gene redesign and heterologous transformation, ketocarotenoids and canthaxanthin content were significantly increased, achieving 0.3% and 0.2% dry weight. Canthaxanthin could be produced using CO2 as the only carbon source at 1.5 mg/L titer. These bkt-engineered lines hold potential for industrial applications in fish or poultry feed sectors, where canthaxanthin and ketocarotenoids are required as pigmentation agents.
Keywords: Nannochloropsis; Canthaxanthin; Carotenoids; Ketocarotenoids; Microalgae.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: Authors declare a competing financial interest: a patent application about the use of the BKT gene has been submitted by the University of Verona and granted at national level (Patent application n. 102021000027824 and PCT/IB2022/060381 “modified β-carotene ketolase (BKT), corresponding nucleic acid and microalgae strain comprising the same) having M.B. and S.C. among inventors. M.B. is the CEO and shareholder of asteasier srl, a company which could commercially exploit the results reported in this paper.
Figures





References
-
- Randhir A, Laird D, Maker G, Trengove R, Moheimani N. Microalgae: a potential sustainable commercial source of sterols. Algal Research-Biomass Biofuels Bioprod. 2020;46.
-
- Priyadarshani I, Rath B, bioactive compounds from microalgae. and cyanobacteria: utility and applications. Int J Pharm Sci Res; 2012. pp. 4123–30.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

