NOTCH2NLC GGC intermediate repeat with serine induces hypermyelination and early Parkinson's disease-like phenotypes in mice
- PMID: 39609868
- PMCID: PMC11603791
- DOI: 10.1186/s13024-024-00780-2
NOTCH2NLC GGC intermediate repeat with serine induces hypermyelination and early Parkinson's disease-like phenotypes in mice
Abstract
Background: The expansion of GGC repeats (typically exceeding 60 repeats) in the 5' untranslated region (UTR) of the NOTCH2NLC gene (N2C) is linked to N2C-related repeat expansion disorders (NREDs), such as neuronal intranuclear inclusion disease (NIID), frontotemporal dementia (FTD), essential tremor (ET), and Parkinson's disease (PD). These disorders share common clinical manifestations, including parkinsonism, dementia, seizures, and muscle weakness. Intermediate repeat sizes ranging from 40 to 60 GGC repeats, particularly those with AGC-encoded serine insertions, have been reported to be associated with PD; however, the functional implications of these intermediate repeats with serine insertion remain unexplored.
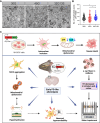
Methods: Here, we utilized cellular models harbouring different sizes of N2C variant 2 (N2C2) GGC repeat expansion and CRISPR-Cas9 engineered transgenic mouse models carrying N2C2 GGC intermediate repeats with and without serine insertion to elucidate the underlying pathophysiology associated with N2C intermediate repeat with serine insertion in NREDs.
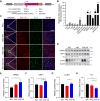
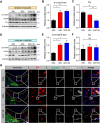
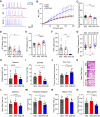
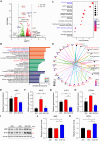
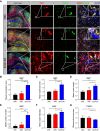
Results: Our findings revealed that the N2C2 GGC intermediate repeat with serine insertion (32G13S) led to mitochondrial dysfunction and cell death in vitro. The neurotoxicity was influenced by the length of the repeat and was exacerbated by the presence of the serine insertion. In 12-month-old transgenic mice, 32G13S intensified intranuclear aggregation and exhibited early PD-like characteristics, including the formation of α-synuclein fibers in the midbrain and the loss of tyrosine hydroxylase (TH)-positive neurons in both the cortex and striatum. Additionally, 32G13S induced neuronal hyperexcitability and caused locomotor behavioural impairments. Transcriptomic analysis of the mouse cortex indicated dysregulation in calcium signaling and MAPK signaling pathways, both of which are critical for mitochondrial function. Notably, genes associated with myelin sheath components, including MBP and MOG, were dysregulated in the 32G13S mouse. Further investigations using immunostaining and transmission electron microscopy revealed that the N2C intermediate repeat with serine induced mitochondrial dysfunction-related hypermyelination in the cortex.
Conclusions: Our in vitro and in vivo investigations provide the first evidence that the N2C-GGC intermediate repeat with serine promotes intranuclear aggregation of N2C, leading to mitochondrial dysfunction-associated hypermyelination and neuronal hyperexcitability. These changes contribute to motor deficits in early PD-like neurodegeneration in NREDs.
Keywords: NOTCH2NLC; AGC interruption; Early Parkinson’s disease; GGC repeat expansion; Hyperexcitability; Hypermyelination; Intermediate repeat; Mitochondrial dysfunction.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Informed consents were obtained from patients. The use of human samples in this study was properly assessed and approved by SingHealth Centralised Institutional Review Board (2017/2602). Animals were maintained under institutional guidelines, and all protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the National Neuroscience Institute (NNI) and Tan Tock Seng Hospital. Consent for publication: Not applicable. Competing interests: The authors report no competing interests.
Figures


References
-
- Okubo M, Doi H, Fukai R, Fujita A, Mitsuhashi S, Hashiguchi S, Kishida H, Ueda N, Morihara K, Ogasawara A, et al. GGC repeat expansion of NOTCH2NLC in adult patients with Leukoencephalopathy. Ann Neurol. 2019;86:962–8. - PubMed
-
- Sone J, Mitsuhashi S, Fujita A, Mizuguchi T, Hamanaka K, Mori K, Koike H, Hashiguchi A, Takashima H, Sugiyama H, et al. Long-read sequencing identifies GGC repeat expansions in NOTCH2NLC associated with neuronal intranuclear inclusion disease. Nat Genet. 2019;51:1215–21. - PubMed
-
- Jiao B, Zhou L, Zhou Y, Weng L, Liao X, Tian Y, Guo L, Liu X, Yuan Z, Xiao X, et al. Identification of expanded repeats in NOTCH2NLC in neurodegenerative dementias. Neurobiol Aging. 2020;89:142. e141-142 e147. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

