Genetic-by-age interaction analyses on complex traits in UK Biobank and their potential to identify effects on longitudinal trait change
- PMID: 39609904
- PMCID: PMC11606088
- DOI: 10.1186/s13059-024-03439-9
Genetic-by-age interaction analyses on complex traits in UK Biobank and their potential to identify effects on longitudinal trait change
Abstract
Background: Genome-wide association studies (GWAS) have identified thousands of loci for disease-related human traits in cross-sectional data. However, the impact of age on genetic effects is underacknowledged. Also, identifying genetic effects on longitudinal trait change has been hampered by small sample sizes for longitudinal data. Such effects on deteriorating trait levels over time or disease progression can be clinically relevant.
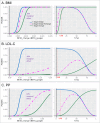
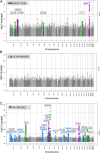
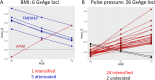
Results: Under certain assumptions, we demonstrate analytically that genetic-by-age interaction observed in cross-sectional data can be indicative of genetic association on longitudinal trait change. We propose a 2-stage approach with genome-wide pre-screening for genetic-by-age interaction in cross-sectional data and testing identified variants for longitudinal change in independent longitudinal data. Within UK Biobank cross-sectional data, we analyze 8 complex traits (up to 370,000 individuals). We identify 44 genetic-by-age interactions (7 loci for obesity traits, 26 for pulse pressure, few to none for lipids). Our cross-trait view reveals trait-specificity regarding the proportion of loci with age-modulated effects, which is particularly high for pulse pressure. Testing the 44 variants in longitudinal data (up to 50,000 individuals), we observe significant effects on change for obesity traits (near APOE, TMEM18, TFAP2B) and pulse pressure (near FBN1, IGFBP3; known for implication in arterial stiffness processes).
Conclusions: We provide analytical and empirical evidence that cross-sectional genetic-by-age interaction can help pinpoint longitudinal-change effects, when cross-sectional data surpasses longitudinal sample size. Our findings shed light on the distinction between traits that are impacted by age-dependent genetic effects and those that are not.
Keywords: Blood pressure; GWAS; Genetic-by-age interaction; Lipids; Longitudinal; Obesity; UK Biobank.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures




Similar articles
-
Distinct explanations underlie gene-environment interactions in the UK Biobank.Am J Hum Genet. 2025 Mar 6;112(3):644-658. doi: 10.1016/j.ajhg.2025.01.014. Epub 2025 Feb 17. Am J Hum Genet. 2025. PMID: 39965571
-
Genetic influence on within-person longitudinal change in anthropometric traits in the UK Biobank.Nat Commun. 2024 May 6;15(1):3776. doi: 10.1038/s41467-024-47802-7. Nat Commun. 2024. PMID: 38710707 Free PMC article.
-
Multi-trait Genome-Wide Analysis Identified 20 Novel Loci for Sarcopenia-Related Traits in UK Biobank.Calcif Tissue Int. 2025 Jan 3;116(1):10. doi: 10.1007/s00223-024-01312-2. Calcif Tissue Int. 2025. PMID: 39751833
-
The UK Biobank: A Shining Example of Genome-Wide Association Study Science with the Power to Detect the Murky Complications of Real-World Epidemiology.Annu Rev Genomics Hum Genet. 2022 Aug 31;23:569-589. doi: 10.1146/annurev-genom-121321-093606. Epub 2022 May 4. Annu Rev Genomics Hum Genet. 2022. PMID: 35508184 Review.
-
Is population structure in the genetic biobank era irrelevant, a challenge, or an opportunity?Hum Genet. 2020 Jan;139(1):23-41. doi: 10.1007/s00439-019-02014-8. Epub 2019 Apr 27. Hum Genet. 2020. PMID: 31030318 Free PMC article. Review.
Cited by
-
Heritability of Alzheimer-related plasma biomarkers in the Amish population.medRxiv [Preprint]. 2025 May 21:2025.05.13.25327557. doi: 10.1101/2025.05.13.25327557. medRxiv. 2025. PMID: 40463532 Free PMC article. Preprint.
-
Polygenic and pharmacogenomic contributions to medication dosing: a real-world longitudinal biobank study.J Transl Med. 2025 Jul 2;23(1):727. doi: 10.1186/s12967-025-06782-y. J Transl Med. 2025. PMID: 40605081 Free PMC article.
References
-
- Venkatesh SS, Ganjgahi H, Palmer DS, Coley K, Wittemans LBL, Nellaker C, et al. The genetic architecture of changes in adiposity during adulthood. medRxiv Prepr Serv Heal Sci. 2023; Available from: http://www.ncbi.nlm.nih.gov/pubmed/36711652%0A; http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC9882550.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

