TbpB-based oral mucosal vaccine provides heterologous protection against Glässer's disease caused by different serovars of Spanish field isolates of Glaesserella parasuis
- PMID: 39609907
- PMCID: PMC11606180
- DOI: 10.1186/s40813-024-00404-7
TbpB-based oral mucosal vaccine provides heterologous protection against Glässer's disease caused by different serovars of Spanish field isolates of Glaesserella parasuis
Abstract
Background: Glaesserella parasuis (G. parasuis) is the primary agent of Glässer's disease, significantly affecting nursery and early fattening piglets. Current prophylactic measures, mainly serovar-specific bacterins administered to sows, are limited by maternal immunity, which can interfere with active immunization in piglets. Subunit vaccines containing G. parasuis-specific antigenic molecules show promise but are not yet commercially available. Transferrin-binding proteins (Tbp), which enable G. parasuis to acquire iron in low-iron environments like mucosal surfaces, have been proposed as potential vaccine antigens. The mucosal administration of a TbpB-based subunit vaccine could provide a promising solution to overcome the limitations posed by maternal immunity, offering an effective approach to control the disease in weaning piglets. This study, conducted in two phases, primarily evaluates (days 0-45) the immunogenicity of a two-dose oral mucosal TbpB-based subunit vaccine (TbpBY167A) administered to colostrum-deprived piglets, and subsequently (days 45-52), its heterologous protection by challenging these piglets with four G. parasuis clinical isolates from different TbpB clusters (I, III) and serovars (SV1, SV4, SV5, SV7) recovered from Spanish pig farms.
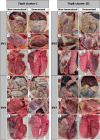
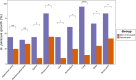
Results: The oral mucosal administration of the two-dose TbpB-based vaccine induced a robust humoral immune response in immunized colostrum-deprived piglets, significantly increasing IgA and IgM concentration 15 days after the second dose (p < 0.01). Upon challenge with four G. parasuis clinical isolates, the vaccine demonstrated heterologous protection, markedly improving survival rates (OR: 8.45; CI 95%: 4.97-14.36) and significantly reducing clinical signs and lesions, regardless of the TbpB cluster and serovar. The vaccine reduced G. parasuis colonization in the respiratory tract (p < 0.0001) and G. parasuis systemic target tissues, like tarsus and carpus joints, liver, and brain (p < 0.05). Immunohistochemical analysis showed a lower macrophage count in different lung locations of immunized piglets (p < 0.0001).
Conclusions: This study demonstrates that oral mucosal administration of the TbpBY167A subunit vaccine in piglets provides effective heterologous protection against diverse virulent European G. parasuis field isolates, significantly reducing bacterial colonization and dissemination. This vaccine offers a promising alternative to traditional bacterins, overcoming limitations due to maternal immunity, and represents a strong candidate for universal vaccination against Glässer's disease.
Keywords: Glaesserella parasuis; Cross-protection; Glässer’s disease; Humoral immune response; Immunization; Needle-free vaccination; Porcine respiratory disease complex; Swine; TbpBY167A subunit vaccine.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures involving animals were approved by the institutional bioethical committee (Reference Number OEBA-ULE-003–2022) and performed according to European regulations regarding animal welfare and protection of animals used for experimental and other scientific purposes. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





References
-
- White M, BVSc, LLB, DPM, MRCVS. Glässers Disease. Available online: https://www.nadis.org.uk/disease-a-z/pigs/glaessers-disease/. Accessed 17 Mar 2024.
-
- Harris DL, Ross RF, Switzer WP. Incidence of certain microorganisms in nasal cavities of swine in Iowa. Am J Vet Res. 1969;30(9):1621–4. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

