Reduced Biofilm Accumulation on Implants Treated With Implantoplasty-An In Situ Trial With a Within-Subject Comparison
- PMID: 39610010
- PMCID: PMC11604595
- DOI: 10.1002/cre2.70043
Reduced Biofilm Accumulation on Implants Treated With Implantoplasty-An In Situ Trial With a Within-Subject Comparison
Abstract
Objectives: This study aimed to evaluate potential differences in biofilm accumulation on three different implant surfaces: turned surface (TS), modified surface (MS), and modified surface treated with implantoplasty (IPS), using a within-subject comparison.
Material and methods: Ten volunteers wore individualized splints containing three titanium implants with different surfaces (TS, MS, and IPS) on each buccal side of the splint. The implant position (anterior, central, and posterior) was randomly assigned among the three implants on each side. Volunteers were instructed to wear the splint for 72 h and to remove it only for eating, drinking, and performing standard oral hygiene; the splint itself was not cleaned. After 72 h, the implants were carefully removed from the splint, and the accumulated biofilm was assessed using a crystal violet assay by measuring intensity/absorbance at 570 nm.
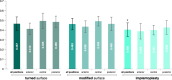
Results: All volunteers reported no deviations from the instructions. The lowest mean amount of biofilm (0.405 ± 0.07) was detected on implants of the IPS group, followed by implants of the MS (0.463 ± 0.06) and TS group (0.467 ± 0.07). A multilevel mixed-effects linear regression analysis confirmed that implants of the IPS group accumulated a significantly lower amount of biofilm than the other surfaces (p < 0.001); however, no significant difference was detected between implants of the TS and MS groups (p = 0.806).
Conclusions: Implantoplasty can generate a surface significantly less conducive to biofilm accumulation in the short term compared to pristine implants with turned or modified surfaces.
Trial registration: clinicaltrials.gov identifier: NCT06049121.
Keywords: biofilm; crystal violet assay; implant surface; implantoplasty; peri‐implantitis.
© 2024 The Author(s). Clinical and Experimental Dental Research published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
The Influence of Implantoplasty on Surface Roughness, Biofilm Formation, and Biocompatibility of Titanium Implants: A Systematic Review.Int J Oral Maxillofac Implants. 2021 September/October;36(5):e111–e119. doi: 10.11607/jomi.8785. Epub 2021 Jun 22. Int J Oral Maxillofac Implants. 2021. PMID: 34157063
-
Modified implant surface with slower and less initial biofilm formation.Clin Implant Dent Relat Res. 2015 Jun;17(3):461-8. doi: 10.1111/cid.12140. Epub 2013 Aug 27. Clin Implant Dent Relat Res. 2015. PMID: 23981274
-
The Effect of Implantoplasty on Fracture Resistance and Implant Surface Changes: An In Vitro and Finite Element Analysis Study.Clin Implant Dent Relat Res. 2025 Feb;27(1):e13409. doi: 10.1111/cid.13409. Epub 2024 Nov 6. Clin Implant Dent Relat Res. 2025. PMID: 39506335
-
Biofilm Formation on Dental Implant Surface Treated by Implantoplasty: An In Situ Study.Dent J (Basel). 2020 May 6;8(2):40. doi: 10.3390/dj8020040. Dent J (Basel). 2020. PMID: 32384621 Free PMC article.
-
Biofilm on dental implants: a review of the literature.Int J Oral Maxillofac Implants. 2009 Jul-Aug;24(4):616-26. Int J Oral Maxillofac Implants. 2009. PMID: 19885401 Review.
References
-
- Bertl, K. , and Stavropoulos A.. 2021. “A Mini Review on Non‐Augmentative Surgical Therapy of Peri‐Implantitis—What Is Known and What Are the Future Challenges.” Frontiers in Dental Medicine 2: 659361.
-
- Bianchini, M. A. , Galarraga‐Vinueza M. E., Apaza‐Bedoya K., De Souza J. M., Magini R., and Schwarz F.. 2019. “Two to Six‐Year Disease Resolution and Marginal Bone Stability Rates of a Modified Resective‐Implantoplasty Therapy in 32 Peri‐Implantitis Cases.” Clinical Implant Dentistry and Related Research 21: 758–765. 10.1111/cid.12773. - DOI - PubMed
-
- Bianchini, M. A. , Kuhlkamp L. F., Schwarz F., and Galarraga‐Vinueza M. E.. 2024. “Clinical and Radiographic Outcomes of Resective Surgery With Adjunctive Implantoplasty Over a 6‐ to 11‐Year Follow‐Up: A Case Series.” International Journal of Periodontics & Restorative Dentistry 44: 466–476. 10.11607/prd.6756. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

