Patient-derived tumor organoid and fibroblast assembloid models for interrogation of the tumor microenvironment in esophageal adenocarcinoma
- PMID: 39610249
- PMCID: PMC11704619
- DOI: 10.1016/j.crmeth.2024.100909
Patient-derived tumor organoid and fibroblast assembloid models for interrogation of the tumor microenvironment in esophageal adenocarcinoma
Abstract
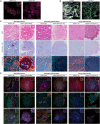
The tumor microenvironment (TME) comprises all non-tumor elements of cancer and strongly influences disease progression and phenotype. To understand tumor biology and accurately test new therapeutic strategies, representative models should contain both tumor cells and normal cells of the TME. Here, we describe and characterize co-culture tumor-derived organoids and cancer-associated fibroblasts (CAFs), a major component of the TME, in matrix-embedded assembloid models of esophageal adenocarcinoma (EAC). We demonstrate that the assembloid models faithfully recapitulate the differentiation status of EAC and different CAF phenotypes found in the EAC patient TME. We evaluate cell phenotypes by combining tissue-clearing techniques with whole-mount immunofluorescence and histology, providing a practical framework for the characterization of cancer assembloids.
Keywords: CP: Cancer biology; CP: Stem cell; assembloids; cancer-associated fibroblasts; esophageal adenocarcinoma; organoids; tumor microenvironment.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Al-Batran S.E., Homann N., Pauligk C., Goetze T.O., Meiler J., Kasper S., Kopp H.G., Mayer F., Haag G.M., Luley K., et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948–1957. doi: 10.1016/s0140-6736(18)32557-1. - DOI - PubMed
-
- Eyck B.M., van Lanschot J.J.B., Hulshof M.C.C.M., van der Wilk B.J., Shapiro J., van Hagen P., van Berge Henegouwen M.I., Wijnhoven B.P.L., van Laarhoven H.W.M., Nieuwenhuijzen G.A.P., et al. Ten-Year Outcome of Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The Randomized Controlled CROSS Trial. J. Clin. Oncol. 2021;39:1995–2004. doi: 10.1200/jco.20.03614. - DOI - PubMed
-
- Bang Y.J., Van Cutsem E., Feyereislova A., Chung H.C., Shen L., Sawaki A., Lordick F., Ohtsu A., Omuro Y., Satoh T., et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/s0140-6736(10)61121-x. - DOI - PubMed
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

