Assessment of the initial results of pituitary tumor registry at a tertiary hospital of Iran: 2009-2022
- PMID: 39610525
- PMCID: PMC11599527
- DOI: 10.1007/s40200-024-01481-9
Assessment of the initial results of pituitary tumor registry at a tertiary hospital of Iran: 2009-2022
Abstract
Objectives: The Pituitary Tumor Registry intends to provide a platform for clinical research and basic sciences, with an emphasis on disease outcomes.
Methods: In this retrospective cohort study, all patient data, including demographics, vital signs, symptoms and signs, medical history, medications, past drug history, paraclinical data, treatment modalities, post-surgery follow-up, treatment responsiveness, and pathology reports, have been gathered from the electronic patient records of the Endocrinology and Metabolism Research Center affiliated with Tehran University of Medical Sciences.
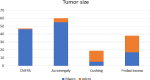
Results: A total of 200 patients with pituitary adenoma were identified. Acromegaly was the most prevalent adenoma, accounting for 35% (n = 70) of cases, followed by clinically nonfunctional pituitary adenoma (CNFPA) at 28.5% (n = 57), prolactinoma at 23% (n = 46), and Cushing disease at 13.5% (n = 27). All of the patients with Cushing disease had surgery, with trans-sphenoidal surgery accounting for 92.59%. Prolactinoma was mostly treated with medication (82.60% of cases). Post-operative complications were reported, including cerebrospinal fluid (CSF) leakage in CNFPA cases (24.13%, n = 7) and diabetes insipidus (DI) in acromegaly (13.46%, n = 7). Unfortunately, 5 patients died: two with acromegaly, two with CNFPA and one with Cushing disease.
Conclusions: This registry permits a comprehensive management, long term follow up and treatment outcomes of patients with pituitary adenomas.
Keywords: Acromegaly; Cushing disease; Non-functional adenoma; Pituitary adenoma; Pituitary tumors; Prolactinoma; Registry.
© The Author(s), under exclusive licence to Tehran University of Medical Sciences 2024. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestOn behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures
References
-
- Aflorei ED, Korbonits M. Epidemiology and etiopathogenesis of pituitary adenomas. J Neurooncol. 2014;117(3):379–94. 10.1007/s11060-013-1354-5. - PubMed
-
- Daly AF, Rixhon M, Adam C, Dempegioti A, Tichomirowa MA, Beckers A. High prevalence of Pituitary adenomas: a cross-sectional study in the Province of Liège, Belgium. J Clin Endocrinol Metabolism. 2006;91(12):4769–75. 10.1210/jc.2006-1668. - PubMed
-
- Khamseh ME, Tehrani MRM, Mousavi Z, Malek M, Imani M, Tehrani NH, et al. Iran pituitary tumor registry: description of the program and initial results. Arch Iran Med. 2017;20(12):746–51. - PubMed
-
- Araujo-Castro M, Berrocal VR, Pascual-Corrales E. Pituitary tumors: epidemiology and clinical presentation spectrum. Hormones. 2020;19(2):145–55. 10.1007/s42000-019-00168-8. - PubMed
-
- Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, et al. The prevalence of pituitary adenomas: a systematic review. Cancer: Interdisciplinary Int J Am Cancer Soc. 2004;101(3):613–9. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous