Greater Trochanteric Pain Syndrome and the Efficacy of Platelet-Rich Plasma Injections: A Systematic Review
- PMID: 39610638
- PMCID: PMC11604237
- DOI: 10.7759/cureus.72597
Greater Trochanteric Pain Syndrome and the Efficacy of Platelet-Rich Plasma Injections: A Systematic Review
Abstract
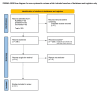
Greater trochanteric pain syndrome (GTPS) is one of the most prevalent causes of lateral hip pain. The incidence rate is as high as 1.8 patients per 1000 annually, with females predominantly affected. We compared and analysed the effectiveness of platelet-rich plasma (PRP) injections in treating GTPS. Literature search was carried out on PubMed, Embase and Cochrane by two independent reviewers using the terms: 'Greater Trochanteric Pain syndrome' and 'Platelet-rich plasma'. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed, and the Cochrane risk of bias tool and Methodological Index for Non-Randomized Studies (MINORS) tool were used to assess bias. Nine studies were shortlisted and reviewed for patient sample size, diagnostic modalities, the presence of tendinopathy or bursitis, the number of PRP injections administered, and the length of symptom relief achieved. We analysed nine studies between 2013 to 2024 comprising of a total of 508 patients who received treatment with PRP injections for lateral hip pain. There was an improvement and sustained relief in symptoms in eight studies, while one reported no change. Many studies indicated PRP injections to be more effective than corticosteroid injections (CSI) in treating GTPS. PRP appears to be an effective injectable treatment option for GTPS, which does not respond to conservative therapy. However, due to the limitations of the current literature, there is a need for more large-scale, high-quality randomized clinical trials to assess further the effectiveness of PRP for treating GTPS.
Keywords: greater trochanteric pain syndrome; gtps; lateral hip pain; platelet rich plasma; prp injections.
Copyright © 2024, Ahmed et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Liberati A, Altman DG, Tetzlaff J, et al. J Clin Epidemiol. 2009;62:0–34. - PubMed
-
- Greater trochanteric pain syndrome: percutaneous tendon fenestration versus platelet-rich plasma injection for treatment of gluteal tendinosis. Jacobson JA, Yablon CM, Henning PT, et al. J Ultrasound Med. 2016;35:2413–2420. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials