Difficult Intubation Alert Is Associated With a Reduced Incidence of Difficult Intubation
- PMID: 39610643
- PMCID: PMC11604021
- DOI: 10.7759/cureus.72625
Difficult Intubation Alert Is Associated With a Reduced Incidence of Difficult Intubation
Abstract
Introduction: Difficult or failed intubation significantly increases the risk of morbidity and mortality. Documentation of a prior difficult or failed tracheal intubation is a strong predictor of future difficult intubation.
Methods: We undertook a quality improvement project to create a redesigned difficult intubation alert with increased visibility in our electronic health record. We sought to determine whether this redesigned alert would be associated with a reduced incidence of difficult intubations. After reviewing many intubation procedure notes, we chose the following criteria to define a predicted future difficult intubation: requiring an awake technique, ease of intubation procedure charted as "difficult" or "unable", procedure requiring flexible bronchoscopy, a procedure requiring three or more attempts, and intubation with a grade three or four view during laryngoscopy. Patients meeting one or more of the above criteria were included in our study. An intervention was implemented which consisted of the introduction of a new difficult intubation alert that could easily be applied to a patient's chart by anyone on the anesthesia team. Further, if the anesthesia clinician filling out the intubation procedure note charted an intubation procedure as "difficult" or "unable", they were prompted by a pop-up asking if difficult intubation should be added to the patient's problem list. If yes was clicked, the electronic alert was activated, and a large red banner appeared. Outcomes included the number of patients who had the difficult intubation label in the pre-intervention period, the number of patients who had the new difficult intubation alert in the post-intervention period, the number of records with ease of intubation procedure charted as "difficult" or "unable", the number of records requiring three or more attempts at intubation, and the number of records with grade three or four view charted at intubation.
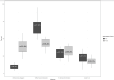
Results: There was an expected increase in the application of the difficult intubation alert from 9% of patients with a difficult intubation label in the pre-intervention period to 38% with the redesigned alert in the post-intervention period which was statistically significant (p<0.001). In the 21 months prior to the introduction of the alert, our screening process identified 988 records as predicted difficult intubations. Of these, 672 (68%) were charted by the intubating clinician as actual difficult intubations with 32% not being recorded as difficult. During the 20 months after the end of the interim period, the screening process identified 976 predicted difficult intubations with intubating anesthesia clinicians charting 416 (42%) of them as actual difficult intubations and 58% found not to be difficult. This reduction in monthly median percent of actual difficult intubations was statistically significant (p<0.001).
Conclusions: The introduction of a difficult intubation alert at our institution was associated with a reduced incidence of difficult intubation.
Keywords: academic anesthesiology; anesthesia documentation; difficult airway intubation; difficult airway management; quality improvement project.
Copyright © 2024, Budde et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. University of Minnesota Institutional Review Board issued approval IRB ID STUDY 00021526. NOT HUMAN RESEARCH Dear Anna Budde, The IRB reviewed the following submission: Type of Review: Initial Study Title of Study: Difficult Airway Banner Quality Improvement Project Investigator: Anna Budde IRB ID: STUDY00021526 Sponsored Funding: None Grant ID: None Internal UMN Funding: None Fund Management Outside University: None IND, IDE, or HDE: None Documents Reviewed with this Submission: • CR3, Category: Other Committee Approvals; • IRB human exemption form-Difficult Airway Banner_02.14.2024.docx, Category: IRB Protocol; The IRB determined that the proposed activity is not research involving human subjects as defined by DHHS and FDA regulations. To arrive at this determination, the IRB used “WORKSHEET: Human Research (HRP-310).” If you have any questions about this determination, please review that Worksheet in the HRPP Toolkit Library and contact the IRB office if needed. Note that this “Not Human Research” determination does not constitute IRB approval or exemption of your activity, but instead simply means that IRB staff have determined that this activity is not subject to the requirements of DHHS and FDA regulated research involving human subjects. Please do not use the terms “IRB approved” or “IRB exempt” when referring to this activity. It is important to understand that you may have other University obligations and requirements. A “Not Human Research” determination is not confirmation that your project has been ethically designed. You are ultimately responsible for ensuring that you understand or have anticipated any ethical concerns related to your project and that you have taken the appropriate steps to eliminate, mitigate or manage those concerns. Ongoing IRB review and approval for this activity is not required; however, this determination applies only to the activities described in the IRB submission and does not apply should any changes be made. If changes are made and there are questions about whether IRB review is required, you must submit a separate, new study ETHOS submission that includes either an updated or newly completed Human Research Determination Form (HRP-503) to the IRB. Sincerely, Jeffery P Perkey, CIP, MLS Senior IRB Analyst. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: Funding: Academic Health Center, University of Minnesota Medical School This research was supported by the National Institutes of Health’s National Center for Advancing Translational Sciences, grant UM1TR004405. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health’s National Center for Advancing Translational Sciences. Conflicts of Interest: None. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- 2022 American Society of Anesthesiologists practice guidelines for management of the difficult airway. Apfelbaum JL, Hagberg CA, Connis RT, et al. Anesthesiology. 2022;136:31–81. - PubMed
-
- Bedside tests for predicting difficult airways: An abridged Cochrane diagnostic test accuracy systematic review. Roth D, Pace NL, Lee A, Hovhannisyan K, Warenits AM, Arrich J, Herkner H. Anaesthesia. 2019;74:915–928. - PubMed
-
- A documented previous difficult tracheal intubation as a prognostic test for a subsequent difficult tracheal intubation in adults. Lundstrøm LH, Møller AM, Rosenstock C, Astrup G, Gätke MR, Wetterslev J. Anaesthesia. 2009;64:1081–1088. - PubMed
-
- Improved difficult airway documentation using structured notes in Anesthesia Information Management Systems. Matava C, Caldeira-Kulbakas M, Chisholm J. Can J Anaesth. 2020;67:625–627. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources