Baricitinib as monotherapy and with topical corticosteroids in moderate-to-severe atopic dermatitis: a systematic review and meta-analysis of dose-response
- PMID: 39610670
- PMCID: PMC11602504
- DOI: 10.3389/falgy.2024.1486271
Baricitinib as monotherapy and with topical corticosteroids in moderate-to-severe atopic dermatitis: a systematic review and meta-analysis of dose-response
Abstract
Introduction: Atopic dermatitis (AD) is a chronic inflammatory skin disorder that affects millions worldwide, presenting challenges in managing symptoms and quality of life. Current treatments include topical corticosteroids (TCS), but novel approaches, such as Janus kinase (JAK) inhibitors, show promise. Baricitinib, a selective JAK1 and JAK2 inhibitor, targets cytokines involved in AD and offers potential benefits beyond traditional therapies.
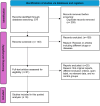
Methods: A systematic review and meta-analysis of randomized controlled trials (RCTs) was performed to evaluate the efficacy and safety of baricitinib in treating moderate-to-severe AD. We followed PRISMA guidelines and assessed data from PubMed, Cochrane Central, ScienceDirect, and ClinicalTrials.gov up to August 2024. The analysis included trials comparing baricitinib to placebo, with or without TCS, evaluating outcomes such as Investigator's Global Assessment (IGA) scores, Eczema Area and Severity Index (EASI) scores, and safety profiles.
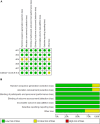
Results: Six RCTs involving 2,595 participants met the inclusion criteria. Baricitinib demonstrated significant improvements in IGA scores, EASI scores, Dermatology Life Quality Index (DLQI), and other outcome measures compared to placebo. The efficacy was consistent across different dosages (1 mg, 2 mg, 4 mg) and whether baricitinib was used with or without TCS. Safety analyses revealed a significant increase in treatment-emergent adverse events (TEAEs), particularly with the 2 mg and 4 mg dosages and with TCS.
Conclusion: Baricitinib, both alone and in combination with TCS, significantly improves symptoms and quality of life in patients with moderate-to-severe AD, with efficacy consistent across dosages. The safety profile is overall acceptable, though a significant increase in TEAEs was observed, particularly with higher dosages and when used with TCS. Ongoing monitoring of TEAEs is recommended, and future trials with longer follow-up periods are suggested to better understand long-term outcomes.
Keywords: JAK inhibitors; atopic dermatitis; baricitinib; eczema area and severity index; treatment-emergent adverse events.
© 2024 Almoghayer, Soomro, Dev, Turesh, Kumar, Kumar, Meghjiani, Lamiya Mir, Hassaan, Qureshi, Kumar, Ashraf, Deepak, Siddiq, Haseeb and Kumar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures













References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

