The role of responsive MRI probes in the past and the future of molecular imaging
- PMID: 39611034
- PMCID: PMC11600131
- DOI: 10.1039/d4sc04849k
The role of responsive MRI probes in the past and the future of molecular imaging
Abstract
Magnetic resonance imaging (MRI) has become an indispensable tool in biomedical research and clinical radiology today. It enables the tracking of physiological changes noninvasively and allows imaging of specific biological processes at the molecular or cellular level. To this end, bioresponsive MRI probes can greatly contribute to improving the specificity of MRI, as well as significantly expanding the scope of its application. A large number of these sensor probes has been reported in the past two decades. Importantly, their development was done hand in hand with the ongoing advances in MRI, including emerging methodologies such as chemical exchange saturation transfer (CEST) or hyperpolarised MRI. Consequently, several approaches on successfully using these probes in functional imaging studies have been reported recently, giving new momentum to the field of molecular imaging, also the chemistry of MRI probes. This Perspective summarizes the major strategies in the development of bioresponsive MRI probes, highlights the major research directions within an individual group of probes (T 1- and T 2-weighted, CEST, fluorinated, hyperpolarised) and discusses the practical aspects that should be considered in designing the MRI sensors, up to their intended application in vivo.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







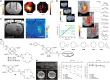

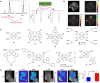



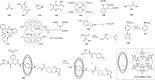





References
-
- Meng Q. Wu M. Shang Z. Zhang Z. Zhang R. Coord. Chem. Rev. 2022;457:214398. doi: 10.1016/j.ccr.2021.214398. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

