A dose escalation study to evaluate the safety of an aerosol BCG infection in previously BCG-vaccinated healthy human UK adults
- PMID: 39611145
- PMCID: PMC11602284
- DOI: 10.3389/fimmu.2024.1427371
A dose escalation study to evaluate the safety of an aerosol BCG infection in previously BCG-vaccinated healthy human UK adults
Abstract
Introduction: Tuberculosis (TB) is the leading cause of death worldwide from a single infectious agent. Bacillus Calmette-Guérin (BCG), the only licensed vaccine, provides limited protection. Controlled human infection models (CHIMs) are useful in accelerating vaccine development for pathogens with no correlates of protection; however, the need for prolonged treatment makes Mycobacterium tuberculosis an unethical challenge agent. Aerosolised BCG provides a potential safe surrogate of infection. A CHIM in BCG-vaccinated as well as BCG-naïve individuals would allow identification of novel BCG-booster vaccine candidates and facilitate CHIM studies in populations with high TB endemicity. The purpose of this study was to evaluate the safety and utility of an aerosol BCG CHIM in historically BCG-vaccinated volunteers.
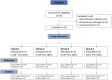
Methods: There were 12 healthy, historically BCG-vaccinated UK adults sequentially enrolled into dose-escalating groups. The first three received 1 × 104 CFU aerosol BCG Danish 1331 via a nebuliser. After safety review, subsequent groups received doses of 1 × 105 CFU, 1 × 106 CFU, or 1 × 107 CFU. Safety was monitored through self-reported adverse events (AEs), laboratory tests, and lung function testing. Immunology blood samples were taken pre-infection and at multiple timepoints post-infection. A bronchoalveolar lavage (BAL) taken 14 days post-infection was analysed for presence of live BCG.
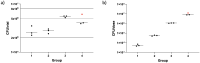
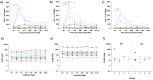
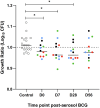
Results: No serious AEs occurred during the study. Solicited systemic and respiratory AEs were frequent in all groups, but generally short-lived and mild in severity. There was a trend for more reported AEs in the highest-dose group. No live BCG was detected in BAL from any volunteers. Aerosol BCG induced potent systemic cellular immune responses in the highest-dose group 7 days post-infection.
Discussion: Aerosol BCG infection up to a dose of 1 × 107 CFU was well-tolerated in historically BCG-vaccinated healthy, UK adults. No live BCG was detected in the BAL fluid 14 days post-infection despite potent systemic responses, suggesting early clearance. Further work is needed to expand the number of volunteers receiving BCG via the aerosol route to refine and establish utility of this aerosol BCG CHIM.
Clinical trial registration: https://clinicaltrials.gov/, identifier NCT04777721.
Keywords: BCG; CHIM; aerosol; tuberculosis; vaccine.
Copyright © 2024 Fredsgaard-Jones, Harris, Morrison, Ateere, Nassanga, Ramon, Mitton, Fletcher, Decker, Preston-Jones, Jackson, Mawer, Satti, Barer, Hinks, Bettinson and McShane.
Conflict of interest statement
The handling editor SF declared a past co-authorship with the author HM. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Organization WH . Global tuberculosis report 2023: Licence: CC BY-NC-SA 3.0 IGO. (2023).
-
- World Health O . The Initiative for Vaccine Research strategic plan 2010-2020. Geneva: World Health Organization; (2010).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

