Heme catabolism and heme oxygenase-1-expressing myeloid cells in pathophysiology
- PMID: 39611159
- PMCID: PMC11604077
- DOI: 10.3389/fimmu.2024.1433113
Heme catabolism and heme oxygenase-1-expressing myeloid cells in pathophysiology
Abstract
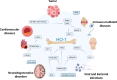
Although the pathological significance of myeloid cell heterogeneity is still poorly understood, new evidence indicates that distinct macrophage subsets are characterized by specific metabolic programs that influence disease onset and progression. Within this scenario, distinct subsets of macrophages, endowed with high rates of heme catabolism by the stress-responsive enzyme heme oxygenase-1 (HO-1), play critical roles in physiologic and pathological conditions. Of relevance, the substrates of HO-1 activity are the heme groups that derive from cellular catabolism and are converted into carbon monoxide (CO), biliverdin and Fe2+, which together elicit anti-apoptotic, anti-inflammatory activities and control oxidative damage. While high levels of expression of HO-1 enzyme by specialized macrophage populations (erythrophagocytes) guarantee the physiological disposal of senescent red blood cells (i.e. erythrocateresis), the action of HO-1 takes on pathological significance in various diseases, and abnormal CO metabolism has been observed in cancer, hematological diseases, hypertension, heart failure, inflammation, sepsis, neurodegeneration. Modulation of heme catabolism and CO production is therefore a feasible therapeutic opportunity in various diseases. In this review we discuss the role of HO-1 in different pathological contexts (i.e. cancer, infections, cardiovascular, immune-mediated and neurodegenerative diseases) and highlight new therapeutic perspectives on the modulation of the enzymatic activity of HO-1.
Keywords: HO-1; immunemetabolism; immunosuppression; innate immunity; myeloid cells.
Copyright © 2024 Consonni, Incerti, Bertolotti, Ballerini, Garlatti and Sica.
Conflict of interest statement
Author MB was employed by the company Navita S.r.l., while authors AS, FC and VG were consultants of Navita S.r.l. These authors declare that the research was conducted in full independence. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could beconstrued as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

