Dexamethasone Acetate-Loaded PLGA Nanospheres Targeting Liver Macrophages
- PMID: 39611304
- PMCID: PMC11827543
- DOI: 10.1002/mabi.202400411
Dexamethasone Acetate-Loaded PLGA Nanospheres Targeting Liver Macrophages
Abstract
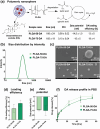
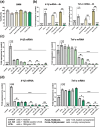
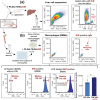
Glucocorticoids are potent anti-inflammatory drugs, although their use is associated with severe side effects. Loading glucocorticoids into suitable nanocarriers can significantly reduce these undesirable effects. Macrophages play a crucial role in inflammation, making them strategic targets for glucocorticoid-loaded nanocarriers. The main objective of this study is to develop a glucocorticoid-loaded PLGA nanocarrier specifically targeting liver macrophages, thereby enabling the localized release of glucocorticoids at the site of inflammation. Dexamethasone acetate (DA)-loaded PLGA nanospheres designed for passive macrophage targeting are synthesized using the nanoprecipitation method. Two types of PLGA NSs in the size range of 100-300 nm are prepared, achieving a DA-loading efficiency of 19 %. Sustained DA release from nanospheres over 3 days is demonstrated. Flow cytometry analysis using murine bone marrow-derived macrophages demonstrates the efficient internalization of fluorescent dye-labeled PLGA nanospheres, particularly into pro-inflammatory macrophages. Significant down-regulation in pro-inflammatory cytokine genes mRNA is observed without apparent cytotoxicity after treatment with DA-loaded PLGA nanospheres. Subsequent experiments in mice confirm liver macrophage-specific nanospheres accumulation following intravenous administration using in vivo imaging, flow cytometry, and fluorescence microscopy. Taken together, the data show that the DA-loaded PLGA nanospheres are a promising drug-delivery system for the treatment of inflammatory liver diseases.
Keywords: PLGA nanospheres; biodegradable nanoparticles; glucocorticoids; liver inflammation; macrophages.
© 2024 The Author(s). Macromolecular Bioscience published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare that no conflict of interest.
Figures


References
-
- Gassler N., Press A., Rauchfuß F., Theis B., Kaemmerer E., AME. Med. J. 2022, 7.
MeSH terms
Substances
Grants and funding
- CZ.02.01.01/00/22_008/0004607/New Technologies for Translational Research in Pharmaceutical Sciences/NETPHARM, co-funded by the European Union
- Project No/Long Term Organization Development Plan 1011 Healthcare Challenges of WMD II of the Military Faculty of Medicine, Hradec Kralove, University of Defence, Czech Republic
- DZRO-FVZ22-ZHN II/Long Term Organization Development Plan 1011 Healthcare Challenges of WMD II of the Military Faculty of Medicine, Hradec Kralove, University of Defence, Czech Republic
- SVV 260 661/Ministerstvo Školství, Mládeže a Tělovýchovy
LinkOut - more resources
Full Text Sources

