Loss of Endothelial APOE4 Dysregulates Neural Function In Vivo
- PMID: 39611383
- PMCID: PMC11681598
- DOI: 10.1161/JAHA.124.035080
Loss of Endothelial APOE4 Dysregulates Neural Function In Vivo
Abstract
Background: We recently found that loss of endothelial cell APOE3 disrupts neurovascular and synaptic function. However, whether endothelial APOE4 is detrimental or protective for neural function under physiological conditions is unknown. Therefore, the goal of this study was to determine the role of endothelial cell APOE4 in regulating brain function in vivo.
Methods and results: We developed APOE4fl/fl/Cdh5(PAC)-CreERT2+/- and APOE4fl/fl/Cdh5(PAC)-CreERT2-/- (control) mice. Knockdown of endothelial cell APOE4 was induced at ≈4 to 5 weeks of age. Experiments were conducted at 9 months of age to evaluate neurovascular and neuronal function via biochemistry, immunohistochemistry, behavior tests, and electrophysiology. Endothelial cell APOE4 knockdown resulted in higher neurovascular permeability, lower claudin-5 vessel coverage, impaired trace fear memory extinction, and disruption of cortical excitatory-inhibitory balance of synaptic activity.
Conclusions: Our data support the novel concept that endothelial cell APOE4 is protective for brain function when other cell types express APOE4.
Keywords: ApoE4; behavior; brain endothelial cells.
Conflict of interest statement
None.
Figures
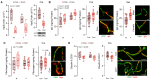

 /
/ P<0.05) from Bonferroni post hoc analysis are color coded: *black for APOE4
Cre+/− vs APOE4
Cre−/− mice or male vs female mice,
P<0.05) from Bonferroni post hoc analysis are color coded: *black for APOE4
Cre+/− vs APOE4
Cre−/− mice or male vs female mice,  dark red for trials 1 to 5 vs other trials within APOE4
Cre−/− or male sex, and
dark red for trials 1 to 5 vs other trials within APOE4
Cre−/− or male sex, and  light red for trials 1–5 vs other trials within APOE4
Cre+/− or female sex. D through G, Data are expressed as a box plot depicting the minimum score, the lower quartile (25%), the median (50%, horizontal line), the upper quartile (75%), maximum values, and mean (+). Data were analyzed using GLM. Asterisks indicating statistical significance (*/
light red for trials 1–5 vs other trials within APOE4
Cre+/− or female sex. D through G, Data are expressed as a box plot depicting the minimum score, the lower quartile (25%), the median (50%, horizontal line), the upper quartile (75%), maximum values, and mean (+). Data were analyzed using GLM. Asterisks indicating statistical significance (*/ /
/ P<0.05) from Bonferroni post hoc analysis are color coded: *black for APOE4
Cre+/− vs APOE4
Cre−/− mice (within a holding potential for D through G),
P<0.05) from Bonferroni post hoc analysis are color coded: *black for APOE4
Cre+/− vs APOE4
Cre−/− mice (within a holding potential for D through G),  dark red is within APOE4
Cre−/−, and <
dark red is within APOE4
Cre−/−, and < light red is within APOE4
Cre+/−. ApoE indicates apolipoprotein E (protein); APOE4; apolipoprotein E4 (gene); APOE4
Cre−/− and Cre‐ indicate APOE4fl/fl/Cdh5(PAC)‐CreERT2−/−; APOE4
Cre+/− and Cre+, APOE4
fl/fl/Cdh5(PAC)‐CreERT2+/−; E‐I, excitatory‐inhibitory; EI, extinction index; F, female; GLM, general linear models; H, habituation; M, male; and PSC, postsynaptic current.
light red is within APOE4
Cre+/−. ApoE indicates apolipoprotein E (protein); APOE4; apolipoprotein E4 (gene); APOE4
Cre−/− and Cre‐ indicate APOE4fl/fl/Cdh5(PAC)‐CreERT2−/−; APOE4
Cre+/− and Cre+, APOE4
fl/fl/Cdh5(PAC)‐CreERT2+/−; E‐I, excitatory‐inhibitory; EI, extinction index; F, female; GLM, general linear models; H, habituation; M, male; and PSC, postsynaptic current.References
-
- Yamazaki Y, Shinohara M, Yamazaki A, Ren Y, Asmann YW, Kanekiyo T, Bu G. ApoE (apolipoprotein E) in brain pericytes regulates endothelial function in an isoform‐dependent manner by modulating basement membrane components. Arterioscler Thromb Vasc Biol. 2020;40:128–144. doi: 10.1161/ATVBAHA.119.313169 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

