Monocyte-derived extracellular vesicles, stimulated by Trypanosoma cruzi, enhance cellular invasion in vitro via activated TGF-β1
- PMID: 39611395
- PMCID: PMC11605483
- DOI: 10.1002/jev2.70014
Monocyte-derived extracellular vesicles, stimulated by Trypanosoma cruzi, enhance cellular invasion in vitro via activated TGF-β1
Abstract
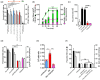
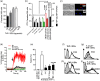
During cell invasion, large Extracellular Vesicle (lEV) release from host cells was dose-dependently triggered by Trypanosoma cruzi metacyclic trypomastigotes (Mtr). This lEV release was inhibited when IP3-mediated Ca2+ exit from the ER and further Ca2+ entry from plasma membrane channels was blocked, but whilst any store-independent Ca2+ entry (SICE) could continue unabated. That lEV release was equally inhibited if all entry from external sources was blocked by chelation of external Ca2+ points to the major contributor to Mtr-triggered host cell lEV release being IP3/store-mediated Ca2+ release, SICE playing a minor role. Host cell lEVs were released through Mtr interaction with host cell lipid raft domains, integrins, and mechanosensitive ion channels, whereupon [Ca2+]cyt increased (50 to 750 nM) within 15 s. lEV release and cell entry of T. cruzi, which increased up to 30 and 60 mpi, respectively, as well as raised actin depolymerization at 60 mpi, were all reduced by TRPC inhibitor, GsMTx-4. Vesicle release and infection was also reduced with RGD peptide, methyl-β-cyclodextrin, knockdown of calpain and with the calpain inhibitor, calpeptin. Restoration of lEV levels, whether with lEVs from infected or uninfected epithelial cells, did not restore invasion, but supplementation with lEVs from infected monocytes, did. We provide evidence of THP-1 monocyte-derived lEV interaction with Mtr (lipid mixing by R18-dequenching; flow cytometry showing transfer to Mtr of R18 from R18-lEVs and of LAP(TGF-β1). Active, mature TGF-β1 (at 175 pg/×105 in THP-1 lEVs) was detected in concentrated lEV-/cell-free supernatant by western blotting, only after THP-1 lEVs had interacted with Mtr. The TGF-β1 receptor (TβRI) inhibitor, SB-431542, reduced the enhanced cellular invasion due to monocyte-lEVs.
Keywords: Trypanosoma cruzi; cell uptake; endocytosis; extracellular vesicles.
© 2024 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors report no conflict of interest.
Figures



References
-
- Ahmed, N. , Riley, C. , Rice, G. E. , Quinn, M. A. , & Baker, M. S. (2002). Alpha(v)beta(6) integrin‐A marker for the malignant potential of epithelial ovarian cancer. The Journal of Histochemistry and Cytochemistry: Official Journal of The Histochemistry Society, 50, 1371–1380. - PubMed
-
- Al‐Nedawi, K. , Meehan, B. , Micallef, J. , Lhotak, V. , May, L. , Guha, A. , & Rak, J. (2008). Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nature Cell Biology, 10, 619–624. - PubMed
-
- Ansa‐Addo, E. , Lange, S. , Stratton, D. , Antwi‐Baffour, S. , Cestari, I. , Ramirez, M. , McCrossan, M. , & Inal, J. (2010). Human plasma membrane‐derived vesicles halt proliferation and induce differentiation of THP‐1 acute monocytic leukemia cells. Journal of Immunology, 185, 5236–5246. - PubMed
-
- Antwi‐Baffour, S. , Malibha‐Pinchbeck, M. , Stratton, D. , Jorfi, S. , Lange, S. , & Inal, J. (2020). Plasma mEV levels in Ghanain malaria patients with low parasitaemia are higher than those of healthy controls, raising the potential for parasite markers in mEVs as diagnostic targets. Journal of Extracellular Vesicles, 9, 1697124. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

