Slow Metabolism-Driven Amplification of Hepatic PPARγ Agonism Mediates Benzbromarone-Induced Obesity-Specific Liver Injury
- PMID: 39611414
- PMCID: PMC11744575
- DOI: 10.1002/advs.202409126
Slow Metabolism-Driven Amplification of Hepatic PPARγ Agonism Mediates Benzbromarone-Induced Obesity-Specific Liver Injury
Abstract
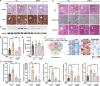
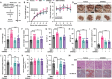
Obesity and nonalcoholic fatty liver disease (NAFLD) are established risk factors for drug-induced liver injury (DILI). The previous study demonstrates that benzbromarone (BBR), a commonly prescribed pharmaceutical agent for managing gout and hyperuricemia, exacerbates hepatic steatosis and liver injury specifically in obese individuals. However, the precise mechanism underpinning this adverse effect remains incompletely elucidated. Given the significance of BBR and its analogs in anti-gout/hyperuricemia drug discovery, elucidating the mechanism by which BBR exacerbates obesity-specific DILI warrants further investigation. In this study, through a combined multi-omics, pharmacological, and pharmacokinetic approaches, it is found that BBR-induced obesity-specific DILI is primarily through the potentiation of peroxisome proliferator-activated receptor gamma (PPARγ) signaling pathways. Further in vivo and in vitro pharmacokinetic analyses reveal that obese db/db mice exhibited a diminished capacity to metabolize BBR in their livers. This reduction leads to prolonged retention of BBR, subsequently resulting in chronic and sustained hepatic PPARγ agonism. This study demonstrates that a slow metabolism-driven amplification of hepatic PPARγ agonism mediates BBR-induced obesity-specific hepatic steatosis and subsequent DILI, which also emphasizes the importance of the reduced hepatic drug metabolism capacity in patients with obesity or pre-existing NAFLD in both clinical practice and drug discovery processes.
Keywords: PPARγ; benzbromarone; drug metabolism; drug‐induced liver injury; obesity.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- a) Palomo L., Mleczko J. E., Azkargorta M., Conde‐Vancells J., González E., Elortza F., Royo F., Falcon‐Perez J. M., Hepatol Commun 2018, 2, 1064; - PMC - PubMed
- b) Galal A. A. A., Ramadan R. A., Metwally M. M. M., El‐Sheikh S. M. A., Ecotoxicol. Environ. Saf. 2019, 171, 502; - PubMed
- c) Upadhyay G., Singh A. K., Kumar A., Prakash O., Singh M. P., Eur. J. Pharmacol. 2008, 596, 146. - PubMed
-
- a) Begriche K., Penhoat C., Bernabeu‐Gentey P., Massart J., Fromenty B., Livers 2023, 3, 33; - PMC - PubMed
- b) Allard J., Le Guillou D., Begriche K., Fromenty B., Adv. Pharmacol. 2019, 85, 75; - PubMed
- c) Kučera O., Roušar T., Staňková P., Haňáčková L., Lotková H., Podhola M., Cervinková Z., J Gastroenterol Hepatol 2012, 27, 323. - PubMed
-
- Heel R. C., Brogden R. N., Speight T. M., Avery G. S., Drugs 1977, 14, 349. - PubMed
-
- a) Lee M. H., Graham G. G., Williams K. M., Day R. O., Drug Saf. 2008, 31, 643; - PubMed
- b) Wagayama H., Shiraki K., Sugimoto K., Fujikawa K., Shimizu A., Takase K., Nakano T., Tameda Y., J Hepatol 2000, 32, 874; - PubMed
- c) Arai M., Yokosuka O., Fujiwara K., Kojima H., Kanda T., Hirasawa H., Saisho H., J Gastroenterol Hepatol 2002, 17, 625. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
