Identification and validation of respiratory subphenotypes in patients with COVID-19 acute respiratory distress syndrome undergoing prone position
- PMID: 39612112
- PMCID: PMC11607308
- DOI: 10.1186/s13613-024-01414-y
Identification and validation of respiratory subphenotypes in patients with COVID-19 acute respiratory distress syndrome undergoing prone position
Erratum in
-
Correction: Identification and validation of respiratory subphenotypes in patients with COVID-19 acute respiratory distress syndrome undergoing prone position.Ann Intensive Care. 2025 Jan 22;15(1):16. doi: 10.1186/s13613-024-01417-9. Ann Intensive Care. 2025. PMID: 39838238 Free PMC article. No abstract available.
Abstract
Background: The heterogeneity of acute respiratory distress syndrome (ARDS) patients is a challenge for the development of effective treatments. This study aimed to identify and characterize novel respiratory subphenotypes of COVID-19 ARDS, with potential implications for targeted patient management.
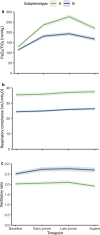
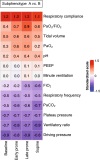
Methods: Consecutive ventilated patients with PCR-confirmed COVID-19 infection, in which prone positioning was clinically indicated for moderate or severe ARDS, were included in a prospective cohort. The patients were assigned to development or validation cohorts based on a temporal split. The PaO2/FiO2 ratio, respiratory compliance, and ventilatory ratio were assessed longitudinally throughout the first prone session. The subphenotypes were derived and validated using machine learning techniques. A K-means clustering implementation designed for joint trajectory analysis was utilized for the unsupervised classification of the development cohort. A random forest model was trained on the labeled development cohort and used to validate the subphenotypes in the validation cohort.
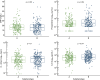
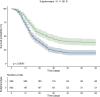
Results: 718 patients were included in a prospective cohort analysis. Of those, 504 were assigned to the development cohort and 214 to the validation cohort. Two distinct subphenotypes, labeled A and B, were identified. Subphenotype B had a lower PaO2/FiO2 response during the prone session, higher ventilatory ratio, and lower compliance than subphenotype A. Subphenotype B had a higher proportion of females (p < 0.001) and lung disease (p = 0.005), higher baseline SAPS III (p = 0.002) and SOFA (p < 0.001) scores, and lower body mass index (p = 0.05). Subphenotype B had also higher levels of the pro-inflammatory biomarker IL-6 (p = 0.017). Subphenotype B was independently associated with an increased risk of 60-day mortality (OR 1.89, 95% CI 1.51-2.36). Additionally, Subphenotype B was associated with a lower number of ventilator-free days on day 28 (p < 0.001) and a lower hospital length of stay (p < 0.001). The subphenotypes were reproducible in the validation cohort.
Conclusion: Our study successfully identified and validated two distinct subphenotypes of COVID-19 ARDS based on key respiratory parameters. The findings suggest potential implications for better patient stratification, risk assessment, and treatment personalization. Future research is warranted to explore the utility of these novel subphenotypes for guiding targeted therapeutic strategies in COVID-19 ARDS.
Keywords: ARDS; Acute respiratory distress syndrome; Covid-19; Machine learning; Mechanical ventilation; Subphenotypes.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Ethics Committee of the National Institute of Infectious Diseases (INI), Oswaldo Cruz Foundation (Fiocruz) on July 3, 2020 (number CAAE: 31050420.8.2001.5262). As this was minimal-risk research using data collected for routine clinical practice, the ethics committee waived the requirement for informed consent. The procedures followed were in accordance with the amended Declaration of Helsinki of 1975. Consent for publication: Not applicable. Competing interests: The authors declare having no competing interests.
Figures
References
-
- WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int. Accessed 20 July 2023.
-
- Bain W, Matute-Bello G. Should we shift the paradigm of preclinical models for ARDS therapies? Thorax. 2019;74(12):1109–10. - PubMed
-
- Enrichment strategies for clinical trials to support determination of effectiveness of human drugs and biological products—Digital Collections—National Library of Medicine. https://collections.nlm.nih.gov/catalog/nlm:nlmuid-101760015-pdf. Accessed 20 July 2023.
-
- Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, ARDS Definition Task Force, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33. - PubMed
LinkOut - more resources
Full Text Sources

