Past innovations and future possibilities in plant chromosome engineering
- PMID: 39612312
- PMCID: PMC11869185
- DOI: 10.1111/pbi.14530
Past innovations and future possibilities in plant chromosome engineering
Abstract
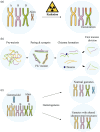
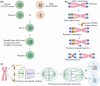
Plant chromosome engineering has emerged as a pivotal tool in modern plant breeding, facilitating the transfer of desirable traits through the incorporation of alien chromosome fragments into plants. Here, we provide a comprehensive overview of the past achievements, current methodologies and future prospects of plant chromosome engineering. We begin by examining the successful integration of specific examples such as the incorporation of rye chromosome segments (e.g. the 1BL/1RS translocation), Dasypyrum villosum segments (e.g. the 6VS segment for powdery mildew resistance), Thinopyrum intermedium segments (e.g. rust resistance genes) and Thinopyrum elongatum segments (e.g. Fusarium head blight resistance genes). In addition to trait transfer, advancements in plant centromere engineering have opened new possibilities for chromosomal manipulation. This includes the development of plant minichromosomes via centromere-mediated techniques, the generation of haploids through CENH3 gene editing, and the induction of aneuploidy using KaryoCreate. The advent of CRISPR/Cas technology has further revolutionized chromosome engineering, enabling large-scale chromosomal rearrangements, such as inversions and translocations, as well as enabling targeted insertion of large DNA fragments and increasing genetic recombination frequency. These advancements have significantly expanded the toolkit for genetic improvement in plants, opening new horizons for the future of plant breeding.
Keywords: CRISPR/Cas technology; Plant chromosome engineering; centromere; minichromosome.
© 2024 The Author(s). Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Plant chromosome engineering - past, present and future.New Phytol. 2024 Jan;241(2):541-552. doi: 10.1111/nph.19414. Epub 2023 Nov 20. New Phytol. 2024. PMID: 37984056 Review.
-
CRISPR/Cas-mediated chromosome engineering: opening up a new avenue for plant breeding.J Exp Bot. 2021 Feb 2;72(2):177-183. doi: 10.1093/jxb/eraa463. J Exp Bot. 2021. PMID: 33258473 Review.
-
Introgression of chromosome segments from multiple alien species in wheat breeding lines with wheat streak mosaic virus resistance.Heredity (Edinb). 2016 Aug;117(2):114-23. doi: 10.1038/hdy.2016.36. Epub 2016 Jun 1. Heredity (Edinb). 2016. PMID: 27245423 Free PMC article.
-
Synthetic minichromosomes in plants: past, present, and promise.Plant J. 2024 Dec;120(6):2356-2366. doi: 10.1111/tpj.17142. Epub 2024 Nov 15. Plant J. 2024. PMID: 39546384 Review.
-
CRISPR-Cas-mediated chromosome engineering for crop improvement and synthetic biology.Nat Plants. 2021 May;7(5):566-573. doi: 10.1038/s41477-021-00910-4. Epub 2021 May 6. Nat Plants. 2021. PMID: 33958776 Review.
Cited by
-
Fine mapping of a novel powdery mildew resistance gene PmDM8 derived from a cultivated emmer (Triticum dicoccum).Theor Appl Genet. 2025 Jun 11;138(7):146. doi: 10.1007/s00122-025-04921-z. Theor Appl Genet. 2025. PMID: 40500408
References
-
- Anderson, G.R. , Papa, D. , Peng, J. , Tahir, M. and Lapitan, N.L. (2003) Genetic mapping of Dn7, a rye gene conferring resistance to the Russian wheat aphid in wheat. Theor. Appl. Genet. 107, 1297–1303. - PubMed
-
- Bao, Y. , Li, X. , Liu, S. , Cui, F. and Wang, H. (2009) Molecular cytogenetic characterization of a new wheat – Thinopyrum intermedium partial amphiploid resistant to powdery mildew and stripe rust. Cytogenet. Genome Res. 126, 390–395. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

