Elevating levels of the endocannabinoid 2-arachidonoylglycerol blunts opioid reward but not analgesia
- PMID: 39612328
- PMCID: PMC11606496
- DOI: 10.1126/sciadv.adq4779
Elevating levels of the endocannabinoid 2-arachidonoylglycerol blunts opioid reward but not analgesia
Abstract
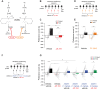
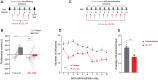
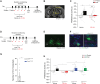
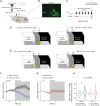
Converging findings have established that the endocannabinoid (eCB) system serves as a possible target for the development of new treatments as a complement to opioid-based treatments. Here, we show in male and female mice that enhancing levels of the eCB, 2-arachidonoylglycerol (2-AG), through pharmacological inhibition of its catabolic enzyme, monoacylglycerol lipase (MAGL), either systemically or in the ventral tegmental area (VTA) with JZL184, leads to a substantial attenuation of the rewarding effects of opioids in mice using conditioned place preference and self-administration paradigms, without altering their analgesic properties. These effects are driven by cannabinoid receptor 1 (CB1R) within the VTA, as VTA CB1R conditional knockout counteracts JZL184's effects. Using fiber photometry with fluorescent sensors for calcium and dopamine (DA), we find that enhancing 2-AG levels diminishes opioid reward-related nucleus accumbens (NAc) activity and DA neurotransmission. Together, these findings reveal that 2-AG diminishes the rewarding properties of opioids and provides a potential adjunctive therapeutic strategy for opioid-related analgesic treatments.
Figures

Update of
-
Elevating levels of the endocannabinoid 2-arachidonoylglycerol blunts opioid reward but not analgesia.bioRxiv [Preprint]. 2024 Apr 2:2024.04.02.585967. doi: 10.1101/2024.04.02.585967. bioRxiv. 2024. Update in: Sci Adv. 2024 Nov 29;10(48):eadq4779. doi: 10.1126/sciadv.adq4779. PMID: 38766079 Free PMC article. Updated. Preprint.
References
-
- Centers for Disease Control and Prevention, WIAQAr Animated Ten Leading Causes of Death. https://wisqars.cdc.gov/animated-leading-causes/.
-
- Strang J., Volkow N. D., Degenhardt L., Hickman M., Johnson K., Koob G. F., Marshall B. D. L., Tyndall M., Walsh S. L., Opioid use disorder. Nat. Rev. Dis. Primers 6, 3 (2020). - PubMed
MeSH terms
Substances
Grants and funding
- R01 DA050454/DA/NIDA NIH HHS/United States
- R01 DA047851/DA/NIDA NIH HHS/United States
- R01 DA042888/DA/NIDA NIH HHS/United States
- R01 MH125006/MH/NIMH NIH HHS/United States
- R01 MH109685/MH/NIMH NIH HHS/United States
- R01 DA049837/DA/NIDA NIH HHS/United States
- R21 DA048635/DA/NIDA NIH HHS/United States
- R33 DA051529/DA/NIDA NIH HHS/United States
- R01 MH118934/MH/NIMH NIH HHS/United States
- R01 DA029122/DA/NIDA NIH HHS/United States
- F30 MH115622/MH/NIMH NIH HHS/United States
- R01 DA053261/DA/NIDA NIH HHS/United States
- R01 DA054368/DA/NIDA NIH HHS/United States
- R01 DA047265/DA/NIDA NIH HHS/United States
- R01 MH123154/MH/NIMH NIH HHS/United States
- R61 DA051529/DA/NIDA NIH HHS/United States
- R01 MH118451/MH/NIMH NIH HHS/United States
- R01 DA042943/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources

