Molecular principles of the assembly and construction of a carboxysome shell
- PMID: 39612341
- PMCID: PMC11606499
- DOI: 10.1126/sciadv.adr4227
Molecular principles of the assembly and construction of a carboxysome shell
Abstract
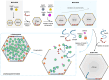
Intracellular compartmentalization enhances biological reactions, crucial for cellular function and survival. An example is the carboxysome, a bacterial microcompartment for CO2 fixation. The carboxysome uses a polyhedral protein shell made of hexamers, pentamers, and trimers to encapsulate Rubisco, increasing CO2 levels near Rubisco to enhance carboxylation. Despite their role in the global carbon cycle, the molecular mechanisms behind carboxysome shell assembly remain unclear. Here, we present a structural characterization of α-carboxysome shells generated from recombinant systems, which contain all shell proteins and the scaffolding protein CsoS2. Atomic-resolution cryo-electron microscopy of the shell assemblies, with a maximal size of 54 nm, unveil diverse assembly interfaces between shell proteins, detailed interactions of CsoS2 with shell proteins to drive shell assembly, and the formation of heterohexamers and heteropentamers by different shell protein paralogs, facilitating the assembly of larger empty shells. Our findings provide mechanistic insights into the construction principles of α-carboxysome shells and the role of CsoS2 in governing α-carboxysome assembly and functionality.
Figures




References
-
- Chen A. H., Silver P. A., Designing biological compartmentalization. Trends Cell Biol. 22, 662–670 (2012). - PubMed
-
- Greening C., Lithgow T., Formation and function of bacterial organelles. Nat. Rev. Microbiol. 18, 677–689 (2020). - PubMed
-
- Vacca I., Insights into bacterial microcompartments. Nat. Rev. Microbiol. 15, 451–451 (2017). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

