Bivalent Omicron BA.1 vaccine booster increases memory B cell breadth and neutralising antibodies against emerging SARS-CoV-2 variants
- PMID: 39612651
- PMCID: PMC11647467
- DOI: 10.1016/j.ebiom.2024.105461
Bivalent Omicron BA.1 vaccine booster increases memory B cell breadth and neutralising antibodies against emerging SARS-CoV-2 variants
Abstract
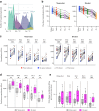
Background: Current literature informs us that bivalent vaccines will generate a broader serum neutralizing antibody response to multiple SARS-CoV-2 variants, but studies on how this breadth relates to the memory B cell (MBC) and T cell responses are sparse. This study compared breadth of neutralising antibody, and memory B and T cell responses to monovalent or a bivalent ancestral/Omicron BA.1 COVID-19 booster vaccine.
Methods: At baseline and 1-month post-booster, neutralisation activity and frequencies of receptor binding domain (RBD)-specific MBCs and Spike-specific memory T cells were measured against a panel of variants.
Findings: Both vaccines boosted neutralising antibodies to 5 variants - Wuhan-Hu-1, Delta, BA.1, BA.5 and JN.1, the latter of which had not yet emerged at the time of sample collection. The bivalent vaccine induced a significantly larger increase in nAb against BA.1 and JN.1. Both vaccines boosted RBD-specific MBC responses to Wuhan-Hu-1, Delta, BA.1 and BA.5 variants with a significantly greater increase for BA.1 in the bivalent group. The breadth of MBCs was significantly higher in those who received the bivalent boost and correlated with nAb breadth. Both vaccines significantly boosted Spike-specific T cell responses to the Wuhan-Hu-1 and BA.5 variants, but only the bivalent vaccine boosted BA.1 responses.
Interpretation: These results suggest that the bivalent vaccine confers an advantage against future novel variants due to increased frequency of broadly reactive RBD-specific B cells.
Funding: Work supported by NSW Health for the NSW Vaccine, Infection and Immunology Collaborative (VIIM).
Keywords: Bivalent vaccine; JN.1; Memory B cells; Omicron BA.1; Receptor binding domain.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The VIIM Collaborative Group of NSW was funded by NSW Health. A.L.C has received NSW Health grant and performed consultancies for Moderna with honoraria directed to his institution. K.J.S. has received honoraria for talks from Moderna. A.K. has grants from NSW Health, NHMRC, MRFF, NIH, Viiv, Unitaid, DFAT, received consulting fee from Merck and Viiv, travel support from HIN STRIVE investigators meeting, performed leadership and advisory role in Board member Scientia Clinical Research Phase 1 Unit, HIV-NAT Advisory Board ASCOT Trial, Chair of DSMB Aegros Advisory Board and pending patent for siRNA for COVID-19. F.B. has received support from NHMRC, Novartis and MRFF. W.J.B. has received support from NSW Vaccine Accelerator, NHMRC, MRFF and NSW Dust Diseases board. I.D.C. has clinical trials support from Eli Lilly and Boehringer. M.W.D has grants from NHMRC and Gilead Australia, payment from Roche Diagnostics for webinar, GSK travel grant and participated as advisor in Gilead and GSK. R.A.B. and R.R. have NHMRC Investigator grants. S.G.Ta has grants from NHMRC, Job Research Foundation and Allegry and Immunology Foundation of Australasia. S.G.Tu has received grants from MRFF, and New South Wales Health COVID-19 Research Grants Round 2.
Figures



References
-
- Yang S., Yu Y., Xu Y., et al. Fast evolution of SARS-CoV-2 BA.2·86 to JN.1 under heavy immune pressure. Lancet Infect Dis. 2023;24(2):e70–e72. - PubMed
-
- Kaku Y., Okumura K., Padilla-Blanco M., et al. Virological characteristics of the SARS-CoV-2 JN.1 variant. Lancet Infect Dis. 2024;24(2):e82. - PubMed
-
- Mak W.A., Visser W., van der Vliet M., Markus H.Y., Koeleman J.G.M., Ong D.S.Y. Ancestral SARS-CoV-2 and Omicron BA.5-specific neutralizing antibody and T-cell responses after Omicron bivalent booster vaccination in previously infected and infection-naive individuals. J Med Virol. 2023;95(8) - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

