Antecedent viral immunization and efficacy of immune checkpoint blockade: an extensive serum antibody profile to predict outcomes in non-small cell lung cancer
- PMID: 39613338
- PMCID: PMC11605809
- DOI: 10.1136/jitc-2024-009931
Antecedent viral immunization and efficacy of immune checkpoint blockade: an extensive serum antibody profile to predict outcomes in non-small cell lung cancer
Abstract
Introduction: Immune checkpoint blockers (ICBs) revolutionized the treatment of patients with advanced non-small cell lung cancer (NSCLC) but only a fraction of them obtain a response, and clinical benefit from these treatments is often difficult to predict. The aim of our study is to unveil the potential implications of antibody response to previous viral infections in predicting response to ICBs in patients with NSCLC.
Methods: Sera from patients treated with ICBs alone, chemotherapy (CT) or a combination of CT-ICBs were analyzed with VirScan (CDI Labs, USA), a high-throughput method that comprehensively analyzes epitope-level antiviral IgG antibodies via programmable phage display and immunoprecipitation sequencing.Total number of unique positive peptides (tUP) was defined as the total number of non-overlapping positive "is a hit" peptides for each patient.
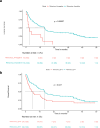
Results: Overall, 387 patients were included. Of them, 129 were treated with ICBs alone, 66 with CT-ICBs and 195 with CT alone. 90 out of 129 patients treated with ICBs alone received ICBs as a subsequent line of treatment, while CT-ICBs and CT were administered as upfront therapies.A higher tUP was correlated with improved overall survival in patients treated with ICBs, and confirmed in the multivariate model (HR 0.43, 95% CI 0.24, 0.79, p=0.006), while it was not in those treated with CT-ICBs (p=0.8) and CT alone (p=0.1).tUP was not correlated with programmed death-ligand 1 (PD-L1) expression, while at the transcriptome level it was correlated with several immune-related pathways, particularly involving B cells.
Conclusion: A higher number of viral peptides recognized by serum antibodies might reflect increased immune fitness, resulting in improved outcomes in ICBs treated patients with NSCLC.
Keywords: Antibody; Biomarker; Immune Checkpoint Inhibitor; Immunization; Lung Cancer.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DP: Consulting, advisory role or lectures: AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, Merck, Novartis, Pfizer, prIME Oncology, Peer CME, Roche. Honoraria: AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Eli Lilly, Merck, Novartis, Pfizer, prIME Oncology, Peer CME, Roche. Clinical trials research: AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, Merck, Novartis, Pfizer, Roche, Medimmun, Sanofi-Aventis, Taiho Pharma, Novocure, Daiichi Sankyo. Travel, Accommodations, Expenses: AstraZeneca, Roche, Novartis, prIME Oncology, Pfizer; LM: Research grant/Funding (self): Bristol Myers Squibb, Boehringer Ingelheim, Amgen, Stilla, Inivata; Advisory/Consultancy: Roche Diagnostics, Takeda; Honoraria (self): Bristol Myers Squibb, Tecnofarma, Roche; Travel/Accommodation/Expenses: Roche, Boehringer Ingelheim, Takeda, AstraZeneca. TH and GR are employee of C.D.I laboratories. MA: Research funding from Sandoz, Amgen, AstraZeneca; Advisory board for Viatris. FB reports other support from AbbVie, ACEA, Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Eisai, Eli Lilly Oncology, F. Hoffmann-La Roche Ltd., Genentech, Ipsen, Ignyta, Innate Pharma, Loxo, Novartis, Medimmune, Merck, MSD, Pierre Fabre, Pfizer, Sanofi-Aventis, and Takeda outside the submitted work. NC-G has provided expertise through participation in scientific advisory boards to AstraZeneca and to Servier and received a research grant from Cytune Pharma, Roche, and Sanofi, although these grants were not on the matter of this manuscript. AM has been a clinical investigator of clinical trials sponsored by and has provided expertise through scientific advisory boards and consulting to the following companies commercializing approved anti-PD-(L)1 immunotherapies: Bristol Myers Squibb, Merck Sharp & Dohme, Roche/Genentech, AstraZeneca, Pfizer/Merck Serono, Sanofi, GlaxoSmithKline. BB reports grants from Chugai Pharma Europe Ltd., Hedera Dx, Genmab A/S, Roche SAS, PharmaMar, AbbVie, MSD France, Daiichi Sankyo Inc., Turning Point Therapeutics Inc., Sanofi, Ellipse Pharma Ltd., Springer Healthcare Ltd., F. Hoffmann-La Roche Ltd., Advanced Accelerator Applications International S.A, Taiho Oncology Inc., Chugai Pharmaceutical Co. Ltd., AstraZeneca, Amgen, Socar Research, Janssen, IPSEN, EISAI, Medscape, Inivata Inc., Genzyme Corporation, Takeda, Ose Immunotherapeutics, Blueprint Medicine, GSK, Pfizer, 4D Pharma, Cergentis, Onxeo, Eli Lilly, BeiGene, F-Star, Da Volterra over the past 5 years. All other authors has no COIs to declare.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
