Thioredoxin 1 moonlights as a chaperone for an interbacterial ADP-ribosyltransferase toxin
- PMID: 39613764
- PMCID: PMC11606950
- DOI: 10.1038/s41467-024-54892-w
Thioredoxin 1 moonlights as a chaperone for an interbacterial ADP-ribosyltransferase toxin
Abstract
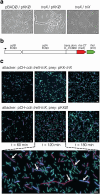
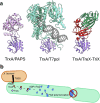
Formation and breakage of disulfide bridges strongly impacts folding and activity of proteins. Thioredoxin 1 (TrxA) is a small, conserved enzyme that reduces disulfide bonds in the bacterial cytosol. In this study, we provide an example of the emergence of a chaperone role for TrxA, which is independent of redox catalysis. We show that the activity of the secreted bacterial ADP-ribosyltransferase (ART) toxin TreX, which does not contain any cysteines, is dependent on TrxA. TreX binds to the reduced form of TrxA via its carboxy-terminal extension to form a soluble and active complex. Structural studies revealed that TreX-like toxins are homologous to Scabin-like ART toxins which possess cysteine residues and form disulfide bridges at the position that superimposes the TrxA binding site in TreX. Our study therefore suggests that thioredoxin 1 evolved alternative functions by maintaining the interaction with cysteine-free substrates.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Braun, V. & Patzer, S. I. Intercellular communication by related bacterial protein toxins: colicins, contact-dependent inhibitors, and proteins exported by the type VI secretion system. FEMS Microbiol. Lett.345, 13–21 (2013). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

